
A more recent article on gastroesophageal reflux in infants and children is available.
Am Fam Physician. 2001;64(11):1853-1861
See patient information handout on gastroesophageal reflux in infants and children, written by the author of this article.
Gastroesophageal reflux is a common, self-limited process in infants that usually resolves by six to 12 months of age. Effective, conservative management involves thickened feedings, positional treatment, and parental reassurance. Gastroesophageal reflux disease (GERD) is a less common, more serious pathologic process that usually warrants medical management and diagnostic evaluation. Differential diagnosis includes upper gastrointestinal tract disorders; cow's milk allergy; and metabolic, infectious, renal, and central nervous system diseases. Pharmacologic management of GERD includes a prokinetic agent such as metoclopramide or cisapride and a histamine-receptor type 2 antagonist such as cimetidine or ranitidine when esophagitis is suspected. Although recent studies have supported the cautious use of cisapride in childhood GERD, the drug is currently not routinely available in the United States.
A common symptom complex in infants is gastroesophageal reflux (GER), which causes parental anxiety resulting in numerous visits to the physician. The etiology of GER has not been well defined.1 In addition to simple parental reassurance and thickened feedings, multiple diagnostic and treatment options are available.
The term GER implies a functional or physiologic process in a healthy infant with no underlying systemic abnormalities. GER is a common condition involving regurgitation, or “spitting up,” which is the passive return of gastric contents retrograde into the esophagus. The prevalence of GER peaks between one to four months of age,2 and usually resolves by six to 12 months of age.3 No gender predilection or definite peak age of onset beyond infancy has been established.4 Regurgitation has been reported in 40 to 65 percent of healthy infants,5 but decreases to 1 percent by one year of age.
| GER | GERD |
|---|---|
| Regurgitation with normal weight gain | Regurgitation with poor weight gain |
| No signs or symptoms of esophagitis | Persistent irritability; pain in infants |
| Lower chest pain, dysphagia, pyrosis in children | |
| Hematemesis and iron deficiency anemia | |
| No significant respiratory symptoms | Apnea and cyanosis in infants |
| Wheezing | |
| Aspiration or recurrent pneumonia | |
| Chronic cough | |
| Stridor | |
| No neurobehavioral symptoms | Neck tilting in infants (Sandifer's syndrome) |
Gastroesophageal reflux disease (GERD) is a pathologic process in infants manifested by poor weight gain, signs of esophagitis, persistent respiratory symptoms, and changes in neurobehavior (Table 1). Abnormal signs and symptoms that warrant a diagnosis of GERD occur in approximately one in 300 infants.6 After the first year of life, GERD is more resistant to complete resolution. A higher prevalence of GERD is present in children who have the following: a history of esophageal atresia with repair7; neurologic impairment and delay8; hiatal hernia9; bronchopulmonary dysplasia10; asthma11; and chronic cough (Table 2). GERD is also associated with pulmonary aspiration, chronic bronchitis, and bronchiectasis.12
Pathophysiology
GASTROINTESTINAL TRACT
In the gastrointestinal (GI) tract, the lower esophageal sphincter is located at the distal end of the esophagus and is under tonic smooth muscle control. Transient lower esophageal sphincter relaxations unassociated with swallowing may be the major mechanism allowing the gastric refluxate to return into the esophagus.1,9,10 Delayed gastric emptying13,14 is another mechanism in infants and older children that predisposes them to gastric distension, increased acid secretion, and esophagitis. Gravitational and positional factors may exacerbate GER and increase the risk of GERD by allowing reflux to occur in a supine position.
RESPIRATORY TRACT
In the respiratory tract, complex reflex responses to the gastric refluxate occur in children by three mechanisms. First, the aspirated material may cause luminal mechanical obstruction. Second, neurally mediated impulses from the refluxate result in local airway or distal esophageal afferent signals stimulating mucous secretion, edema, and bronchial smooth muscle contraction.15 Third, aspiration stimulates the chemical release of inflammatory mediators that cause further respiratory luminal obstruction. These responses can result in signs of upper airway (apnea, stridor, laryngomalacia) and lower airway (chronic cough, wheezing) obstruction. In infants, activation of laryngeal chemoreflexes associated with regurgitation of gastric contents into the pharynx may be associated with episodic prolonged apnea.16
| Esophageal atresia with repair |
| Neurologic impairment and delay |
| Hiatal hernia |
| Bronchopulmonary dysplasia (preterm infants with lung disease) |
| Asthma |
| Cystic fibrosis |
Clinical Manifestations
INFANTS
Infants with GER regurgitate without any secondary signs or symptoms of inadequate growth, esophagitis, or respiratory disease. Infants with GER are thriving and represent the majority of infants who present to the physician with this condition.
Patients with GERD may manifest persistent regurgitation with secondary poor weight gain and failure to thrive.17 Failure to thrive occurs when caloric intake is less than ongoing losses. Other infants may manifest signs of esophagitis, including persistent irritability, pain, feeding problems, and iron deficiency anemia. A subset of infants may demonstrate significant reflux by esophageal pH monitoring but will not have symptoms of regurgitation, known as “silent” GERD.14 All infants with GERD, therefore, do not visibly regurgitate, and the majority of infants who regurgitate do not have GERD.
A variety of respiratory symptoms occur in infants. Apnea with cyanotic episodes may occur secondary to upper airway stimulation by pharyngeal regurgitation, as previously described. Instead of a pure obstructive apnea pattern, a mixed pattern of both obstructive and central types generally predominates. A well-defined relationship between apnea secondary to GERD and an apparent life-threatening event has not been established.10 Another sign of upper airway disease is recurrent stridor. Lower airway symptoms secondary to bronchoconstriction and airway inflammation include wheezing and chronic cough. Aspiration of refluxate may lead to pneumonia, especially in infants with neurologic impairment.
| Affected system | Signs or symptoms | Diagnostic studies | |
|---|---|---|---|
| Gastrointestinal | |||
| Pyloric stenosis | Nonbilious projectile vomiting | Abdominal US or UGI | |
| Malrotation | Bilious vomiting, abdominal distension | UGI and/or contrast enema | |
| Cow's milk allergy | Vomiting, diarrhea, eczema, urticaria | Milk-free diet and milk challenge | |
| Peptic ulcer disease | Epigastric pain and/or nausea | Endoscopy and Helicobacter pylori testing | |
| Hepatitis | Jaundice and right upper quadrant pain | Hepatitis serology and liver function tests | |
| Viral gastroenteritis | Vomiting, diarrhea, fever | None usually required | |
| Urinary tract | |||
| Infection | Vomiting, fever in infants | Urine culture, urinalysis | |
| Obstruction | Abdominal mass, failure to thrive | Renal US and VCUG | |
| Central nervous system | |||
| Hydrocephalus | Vomiting, increased head size | Head computed tomography | |
| Meningitis | Fever, lethargy, vomiting | CSF studies/culture | |
| Metabolic disorders | |||
| Renal tubular acidosis | Vomiting, failure to thrive | Electrolyte panel | |
| Hyperchloremic, normal gap acidosis | Urinalysis for urine pH | ||
| Urea cycle defects | Poor feeding, lethargy, hypotonia | Serum ammonia (NH4+) | |
| Hypocalcemia | Apnea, poor feeding, tetany, seizures | Calcium, phosphate, parathyroid hormone | |
| Drugs/toxins | Vomiting, lethargy, ingestion history | Urine and serum drug screen | |
| Respiratory | Wheezing, cough, stridor | Dependent on history and examination | |
| Functional | Rumination, anorexia | Psychiatric evaluation | |
Finally, abnormal hyperextension of the neck with torticollis (Sandifer's syndrome) may be seen solely in infants with more severe GERD. This movement is perhaps a protective mechanism of an infant with acidic reflux causing esophagitis.
CHILDREN
After infancy, more classic symptoms of esophagitis predominate, including lower chest pain, heartburn (pyrosis), odynophagia, dysphagia, and signs of anemia and esophageal obstruction from stricture formation.17 With the exception of apnea, older children experience respiratory symptoms similar to infants. Complications of reflux esophagitis may be seen, including signs of peptic stricture and Barrett's esophagus, which is the progressive replacement of distal eroded squamous mucosa with metaplastic gastric epithelium. Barrett's esophagus may increase the risk of esophageal adenocarcinoma in adulthood, but the risk is much lower in children.10
Differential Diagnosis of GERD
Other GI and systemic disorders must first be excluded before considering GERD as the main cause of an infant's or child's symptoms of silent or visible regurgitation or vomiting (Table 3). Additional upper GI disorders that require diagnostic consideration include pyloric stenosis, hiatal hernia, pyloric and antral webs, malrotation, hepatitis, and peptic ulcer disease.18 Cow's milk allergy should be strongly considered, especially with increasing evidence of an association between GERD and cow's milk allergy.19 Urinary tract infections and structural defects such as hydronephrosis should be a consideration because patients with these conditions may present with vomiting. Patients with neurologic diseases such as hydrocephalus and meningitis may also present with persistent vomiting. Finally, metabolic disorders such as renal tubular acidosis, urea cycle defects, and hypocalcemia also require consideration. Functional vomiting disorders may coexist with GERD and require a complete psychologic evaluation in addition to conventional medical treatment.
Diagnostic Evaluation
In most cases of GER, no diagnostic study is required. Although scintigraphy may best quantify gastric emptying or aspiration, it is not as commonly used as the upper GI examination (barium fluoroscopy), the esophageal 24-hour pH probe, or the endoscopy with esophageal biopsy. No single definitive study can diagnose GERD. Consultation with a pediatric gastroenterologist may be necessary to select the most appropriate study for individual patients. Table 4 describes the benefits and limitations of each study.
| Study | Advantages | Disadvantages |
|---|---|---|
| Upper GI (Barium fluoroscopy) | Readily available Evaluates upper GI structure | Inadequate screen for GERD |
| Results are operator dependent | ||
| 24-hour pH probe | Quantification of reflux Evaluates atypical symptoms Monitors medical treatment | Requires overnight hospitalization |
| Requires special equipment and trained personnel | ||
| Endoscopy with biopsy | Evaluates persistent GERD, PUD, H. pylori infection, allergic enteropathy, and Barrett's esophagus | Invasive and requires sedation |
UPPER GI EXAMINATION
Upper GI examination is best utilized to identify anatomic abnormalities that may present with symptoms similar to those of GERD. It can identify structural defects such as hiatal hernias, pyloric stenosis, malrotation, antral webs, or even more distal lesions such as intestinal atresia and stenosis.8 This study is more descriptive than quantitative. The importance of reflux demonstrated by this study is not well defined.7 In addition, the upper GI examination lacks adequate sensitivity and specificity to screen for GERD.4
ESOPHAGEAL 24-HOUR PH PROBE MONITORING
The 24-hour pH probe monitoring may be considered the gold standard test for quantitating reflux and for evaluating atypical symptoms such as apnea, stridor, or cough. Calibrated electrodes are placed in the distal esophagus to detect pH changes below 4.0.20 The study measures the number of episodes that last longer than five minutes with pH less than 4.0, the duration of the longest episode, and the percentage of total duration in which pH is below 4.0 (the reflux index). A summary of recommendations for esophageal pH monitoring has been described.21 Another use of the pH probe monitor is for assessment of medical therapy in cases of severe, intractable GERD. The pH probe test requires a short hospital stay and standardized technique and interpretation parameters by a subspecialist team in each medical center.
ENDOSCOPY AND ESOPHAGEAL BIOPSY
Endoscopy with biopsy may be useful to evaluate GERD that is unresponsive to medical therapy. Endoscopy is useful in evaluating symptoms of pain, dysphagia, and hematemesis, and to differentiate GERD from peptic ulcer disease, Helicobacter pylori infection, gastritis, and duodenitis.4 Histopathologic assessment of esophageal mucosa may be performed to grade the severity of esophagitis and detect early Barrett's esophagus. Histopathology may help demonstrate if an eosinophilic enteropathy may be present.
Management
CONSERVATIVE
Conservative treatment for mild symptoms of GER involves thickened feedings and positional changes in infants, and dietary modification in children. Healthy infants who regurgitate without signs of GERD may be managed by thickening feedings with up to one tablespoon of dry rice cereal per 1 oz of formula.3,17 Thickened feeding reduces regurgitation and fussiness, and increases daily caloric intake. Smaller, more frequent feedings are recommended in older infants and children. Furthermore, avoidance of foods and behaviors that decrease lower esophageal sphincter tone should be initiated. This includes excessive intake of caffeinated, acidic, and alcoholic beverages in children and cigarette smoking in adolescents.
Completely upright and prone positioning is beneficial in infants with GERD. These select infants may be exempt from the American Academy of Pediatrics' statement against prone positioning for sleep.17 Soft bedding materials should be avoided in this setting. Prone positioning is not routinely recommended as first-line management of simple regurgitation without evidence of GERD.3 Placing these infants in the supine position is routinely recommended. Seated positioning should be minimized because it provokes reflux by increasing intra-abdominal pressure.
Parents must be assured that most infants with regurgitation and GER respond well to conservative management. Parents should be informed of the widespread prevalence of functional GER in infancy, especially among one- to four-month-olds. Observation of feeding behavior and the interaction between the parent and child is important, and revised instructions on feeding techniques may be necessary.
Because an allergy to cow's milk may manifest with symptoms similar to those of GER, a two week trial of casein hydrolysate formula may be considered17 if patients do not show improvement with conservative measures.19 Caution should be exercised in changing from traditional lactose-based formula to soy formula, because up to 20 percent of infants who have milk protein allergy also demonstrate sensitivity to soy formula.
PHARMACOLOGIC MANAGEMENT
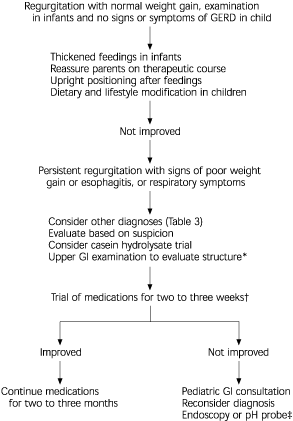
If conservative therapy and a trial of casein hydrolysate formula do not improve symptoms and other differential diagnoses have been considered (Figure 1), medical therapy is likely warranted. One algorithm7 allows for a trial of medical therapy before any diagnostic evaluation is performed. If the patient improves with the use of medication, no further evaluation may be necessary. However, if no improvement occurs, a diagnostic work-up should be performed. It is debatable whether medical therapy should be initiated before diagnostic evaluation or vice-versa.17
| Agent | Dosage | Side effects |
|---|---|---|
| Cimetidine (Tagamet) | 10 mg per kg per dose, four times daily | Headaches, dizziness, diarrhea, gynecomastia |
| Ranitidine (Zantac) | 1 to 2 mg per kg per dose, two to three times daily | Headaches and malaise |
| Metoclopramide (Reglan) | 0.1 mg per kg per dose, four times daily | Drowsiness, restlessness, dystonic reaction, extrapyramidal symptoms |
| Cisapride (Propulsid)* | 0.2 mg per kg per dose, three to four times daily | Cardiac arrhythmia, diarrhea |
An upper GI examination may be the most appropriate study if there is a concern about anatomic defects, especially if a prokinetic agent will be administered. Empiric medical therapy may be initiated if the following conditions are present: adequate suspicion of GERD; the family has been advised of the potential limitations of the upper GI study; minimal suspicion for anatomic defects; and other differential diagnoses have been excluded. The medications used when GERD is suspected include H2-receptor antagonists, prokinetic agents, and proton pump inhibitors such as omeprazole (Prilosec) or lansoprazole (Prevacid) for patients with persistent esophagitis. Lansoprazole is also available in a liquid alkaline form for use in the childhood population.
H2-Receptor Antagonists
Cimetidine (Tagamet) administered in a dosage of 40 mg per kg per day over 12 weeks has been shown to be effective in children with mild to moderate histologically proven esophagitis.22 The recommended starting dosage is 10 mg per kg per dose four times daily7,17 before meals and at bedtime for eight weeks. Potential side effects include headaches, dizziness, diarrhea, and gynecomastia (Table 5).
Ranitidine (Zantac) at 1 to 2 mg per kg per dose two to three times daily (2 to 6 mg per kg per day) is generally recommended as the starting dosage, depending on the severity of symptoms. Higher dosages of 6 to 10 mg per kg per day have successfully healed esophagitis in 75 to 95 percent of children aged three months to 16 years.22 Potential side effects include headaches and malaise, but ranitidine has fewer overall central nervous system and anti-androgenic side effects, compared with cimetidine (Table 5). Famotidine (Pepcid) has no significant role in the management of GERD in the childhood population.
Prokinetic Agents
The two main prokinetic agents used in modern therapy of GERD are metoclopramide (Reglan) and cisapride (Propulsid). However, bethanecol (Urecholine) and domperidone are important for historical reasons. Bethanecol is a cholinergic agonist with mixed clinical efficacy and a potential for exacerbating bronchospasm.10 Domperidone is a peripheral dopamine antagonist with no proven efficacy.4
Metoclopramide is a dopamine antagonist that increases lower esophageal sphincter pressure and improves gastric emptying. Because dopamine receptors are present in the central nervous system, pronounced side effects may include drowsiness, restlessness and, most importantly, dystonic reactions and extrapyramidal movements, especially in infants younger than six months of age.10 The recommended starting dosage is 0.1 mg per kg four times daily before meals and at bedtime (Table 5).
Cisapride is a noncholinergic, nondopaminergic agent that may still be the prokinetic of choice for GERD. It increases the release of acetylcholine from postganglionic nerve endings as a 5-HT4-receptor agonist and increases lower esophageal sphincter pressure and esophageal contractile amplitude.23 Cisapride improves antroduodenal contraction and symptoms of regurgitation, and decreases reflux-associated respiratory symptoms in patients with chronic asthma and bronchopulmonary dysplasia.24 Its efficacy is variable when applied to functional pseudo-obstruction, and should be used with caution in premature neonates younger than 36 weeks of gestation because of the immaturity of the metabolic cytochrome P450 3A4 enzyme complex.25
Reports of fatal arrhythmias associated with the use of cisapride have emerged in the past two years. In a prospective study26 of 35 children between the ages of five months and 18 years who were given cisapride, 11 (31 percent) had evidence of a prolonged QTc greater than 450 msec. Two of these 11 patients had documented torsades de pointes ventricular tachycardia. Both children were receiving cisapride and a macrolide antibiotic that competes with the hepatic cytochrome P450 3A4 enzyme.
In another study27 of 30 infants and children, there was no significant difference in corrected QT intervals during prolonged cisapride therapy at 0.8 mg per kg per day. A consensus statement25 on the role and dosage of cisapride was introduced in 1999 with specific advisable precautions (Table 625). Recently, a prospective study28 of 100 infants given cisapride at 1.0 mg per kg per day demonstrated no significant increase in the QTc interval, except two infants who had an increased QTc interval without evidence of arrhythmia or conductive defect by serial electrocardiogram (ECG). This study supported reconsideration of the use of cisapride in young infants with concomitant adequate parental education. The physician should educate parents concerning the proper dosaging of cisapride, provide a list of drugs to avoid, and document serial ECG monitoring while the child is receiving medication.
| Total dose should not exceed 0.8 mg per kg per day. |
| Avoid concomitant use of macrolides, such as erythromycin, azithromycin (Zithromax), and clarithromycin (Biaxin), and azole antifungals such as ketoconazole (Nizoral). |
| Do not use in patients with a previous history of dysrhythmias or electrolyte disturbances. |
| Use with caution in premature infants with immature cytochrome P450 3A4 activity. |
| Electrocardiogram monitoring while receiving treatment is required. |
| Parental education about proper dosing and drug interactions |
Most of the adverse events associated with cisapride occurred in patients who were taking other medications with potential interactions or those suffering from underlying conditions known to increase the risk of cardiac arrhythmias.29 On July 14, 2000, Janssen Pharmaceutica, Inc., discontinued marketing cisapride (Propulsid) in the United States. A limited access program for cisapride has become available to appropriate patients for whom other therapies are not effective and who meet clearly defined eligibility criteria. These criteria have been established by the manufacturer in collaboration with the U.S. Food and Drug Administration.