
Am Fam Physician. 2001;63(4):697-703
Frequently ignored by the medical community, chronic vulvovaginal symptoms are relatively common and can be frustrating for patients and physicians. Establishing a proper diagnosis will lay the foundation for an effective therapeutic plan. Fungal cultures are an important component of the work-up. The most common causes of chronic vaginal symptoms are recurrent vulvovaginal candidiasis (RVVC), vulvar vestibulitis syndrome and irritant dermatitis. In patients with RVVC caused by Candida albicans, host factors may play an important role. Long-term oral antifungal therapy will break the pattern of recurrence in many patients. Infections caused by other species of yeast may be more resistant to standard treatment approaches.
For any physician involved in the health care of women, the treatment of vaginitis remains a challenge. Vaginitis accounts for more than an estimated 10 million physician office visits annually, and it remains the most common reason for patient visits to obstetrician-gynecologists.1
Over-the-counter (OTC) antifungal therapies now rank among the top 10 best-selling OTC products in the United States with an estimated $250 million in sales annually. Given that millions of women contract vaginitis, it is not surprising that a subpopulation of women with chronic vaginitis (defined here as some type of chronic vulvar or vaginal symptom lasting more than six months) exists. In the past few decades, centers devoted to evaluating and treating women with chronic vaginitis have been established at various academic medical centers. In conjunction with the establishment of these centers, studies of chronic vaginitis have been undertaken and new approaches to the problem have been investigated. The purpose of this article is to summarize the current concepts in the evaluation of women with chronic vulvovaginal symptoms and to discuss recurrent vulvovaginal candidiasis (RVVC) in detail.
Establishing a Diagnosis
Despite the extensive use of medications to treat vaginal symptoms, the appropriateness of their use is undermined by an attitude that deems an accurate diagnosis unnecessary. Women are encouraged to self-medicate, and women with chronic vaginal symptoms often do. In one survey2 of 105 women with chronic vaginal symptoms, 73 percent self-treated with OTC products and 42 percent used alternative medicines. Most of these women thought they had RVVC but, when they were evaluated at a specialty referral center, only 28 percent were diagnosed with RVVC. They were, therefore, using these medications inappropriately and, in at least the 15 percent of women in the study who had irritant dermatitis, their self-treatment played a role in the perpetuation of their symptoms.
Estimates of how accurately women are able to self-diagnose RVVC are difficult to obtain. Initial data3 suggested that women with a prior diagnosis of vulvovaginal candidiasis were able to make an accurate diagnosis up to 82 percent of the time based on symptoms alone. However, this number may represent an overestimate because patients in this study had been initially screened by telephone and were only evaluated and included in the study if they had symptoms consistent with vulvovaginal candidiasis. A recent questionnaire study4 of 634 women found that only 11 percent were able to accurately recognize the classic case scenario for yeast infections. Women who had a previous diagnosis of vulvovaginal candidiasis were also inaccurate in their self-diagnosis, but they were more likely to self-treat. Finally, it should be noted that, even with a strict telephone triage protocol, diagnosis by telephone is only marginally better then random chance.5
When considering the results of these studies, it is apparent that the best chance for making an accurate diagnosis remains with the clinician. Table 1 lists differential diagnoses with the pertinent features of the histories and physical examinations. Particularly in women with chronic vaginitis, timely evaluation during acute exacerbations when the patient is not using any treatment regimen can yield valuable insights into the cause of the symptoms. When taking the medical history, it is important to understand that the symptoms related to vaginitis include a broad spectrum of manifestations, which go well beyond changes in the vaginal discharge. A thorough history should include questions about the nature, quantity and color of the discharge, as well as about irritation, itching, burning and dyspareunia.
| Diagnosis | Symptoms | Findings | Treatment/comments |
|---|---|---|---|
| Recurrent vulvovaginal candidiasis | Itching Irritation Burning Dyspareunia Abnormal discharge | Erythema of vulva and/or vagina Swelling of labia minora Vaginal thrush Normal pH Hyphae/blastospores on microscopy Positive fungal culture | Treatment depends on species of infecting organism; see text for full discussion. |
| Irritant dermatitis | Irritation Burning | Erythema of vulva and/or vestibule Normal pH Negative microscopy Negative fungal culture | Removal of potential irritants Topical corticosteroid ointment |
| Vulvar vestibulitis | Often minor irritation or burning in day-to-day activities Acquired dyspareunia with intromission Pain with other contact with introitus (e.g., tampon insertion) | Areas of focal vestibular erythema Tenderness with palpation of erythematous areas Normal pH Normal microscopy Negative fungal cultural | Removal of potential irritants Topical 4% lidocaine prior to coitus Low-potency topical corticosteroid ointment Low-dose tricyclic antidepressant therapy Pelvic floor biofeedback therapy Low oxalate diet with calcium citrate pills Vestibulectomy with vaginal advancement |
| Bacterial vaginosis | Chronic gray discharge Fishy odor Mild irritation or itching | Gray or yellow discharge ≥pH 4.5 Positive amine test Epithelial cells with more than 20% clue cells | Oral metronidazole (Flagyl), 500 mg twice daily for seven days Topical metronidazole gel 0.75%, at bedtime for five days Topical clindamycin 2% vaginal cream (Cleocin Vaginal) at bedtime for three days No clear consensus on management of patient with recurrent infection; treating partner not shown to be effective. |
| Physiologic discharge | Chronic white discharge, unchanged with past therapies Mild acid odor | Flocculent or thick white discharge Normal pH Normal microscopy Negative fungal culture | None |
Further questions about the primary location of symptoms (vulva, introitus or deep vaginal), variation of symptoms with the menstrual cycle, attempts at self-treatment and response to prior therapies will further clarify the clinical history. Finally, information should be obtained about whether the patient is sexually active, whether she experiences dyspareunia, the number of past and present sex partners, the extent and types of sexual activity and the age at which she first became sexually active.
The physical examination should begin with an inspection of the vulva, looking for areas of erythema, edema, ulceration or chronic vulvar skin changes, combined with palpation using a cotton-tip applicator to elicit areas of tenderness. After insertion of the speculum, the vagina and cervix should be inspected thoroughly and specimens obtained from the lateral vaginal wall for laboratory evaluation. These evaluations should include a vaginal pH, the amine (whiff) test, saline and 10 percent potassium hydroxide (KOH) smears for microscopic examination, and fungal cultures. A normal pH (less than 4.5) effectively rules out bacterial vaginosis, whereas a pH greater than 4.5 has a limited differential diagnosis (Table 2). A whiff test for the presence of amines should be performed by placing a drop of 10 percent KOH onto the vaginal secretions and checking for a fishy odor. Saline microscopy permits identification of trichomonads and clue cells, as well as other additional information.
| Physiologic causes |
| Menses |
| Heavy cervical mucus (i.e., ovulation) |
| Recent intercourse with semen in vagina |
| Pregnancy with rupture of membranes |
| Hypoestrogenism |
| Infectious causes |
| Trichomoniasis |
| Bacterial vaginosis |
| Foreign body with secondary infection |
| Streptococcal vaginitis (group A) (rare) |
| Desquamative inflammatory vaginitis (rare) |
In patients with thinning of the vagina from a lack of estrogen or from infection, parabasal cells may be present. These cells are smaller and more oval with a relatively large nucleus compared with normal vaginal superficial cells. Although white blood cells can be present in normal secretions, a ratio of white blood cells to epithelial cells of more than 1:1 suggests an underlying infection. An assessment of bacterial flora (lactobacillus-dominant or not) can be helpful in determining whether a patient has bacterial vaginosis. By adding 10 percent KOH to the slide, the epithelial cells undergo lysis, which increases the ability to identify hyphae or blastospores (Figure 1)
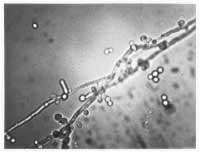
In the general population, approximately 15 to 20 percent of women are asymptomatically colonized with yeast.6 Routine fungal cultures will, therefore, frequently identify women harboring yeast species as part of their flora but who do not require therapy. However, in women with chronic vaginal symptoms, fungal cultures performed on a routine basis are helpful because they corroborate a diagnosis of RVVC, permit identification of the infecting species and increase the 50 percent sensitivity of the 10 percent KOH preparation. In this patient population, successful treatment of a positive fungal culture, corroborated by a negative follow-up culture results in resolution of symptoms approximately 90 percent of the time.7
Some patients may need additional studies. In patients with vulvar or vaginal ulcerations, herpes cultures are indicated. If chronic skin changes occur or ulcers exist, a vulvar biopsy may help identify lichen sclerosus, squamous epithelial hyperplasia, other vulvar dermatoses, or even cancer and its precursors. Cultures for trichomoniasis, gonorrhea and chlamydia are indicated if the secretions reveal many white blood cells but no underlying cause is found.
Using this diagnostic approach, the majority of women with chronic vaginitis can be accurately diagnosed. Table 1 lists the most common diagnoses in women seen at a vaginitis referral center, along with appropriate treatments.2 RVVC remains the most common problem encountered in such centers, although the proportion of women with this condition (28 percent) is smaller than might be expected. Furthermore, the two other most common vaginitides, bacterial vaginosis and trichomoniasis, occur less often than might be expected in this referral population. Patients diagnosed with simple physiologic discharge (7 percent) were seen almost as often as patients diagnosed with bacterial vaginosis (11 percent). Metronidazole-resistant trichomoniasis was diagnosed in fewer than 1 percent of patients. The preponderance of patients who present with chronic vulvar disorders emphasizes the need to broaden the differential diagnosis when evaluating women with suspected chronic vaginitis.
Recurrent Vulvovaginal Candidiasis
Vulvovaginal candidiasis is a common condition. An estimated 75 percent of all women will develop a yeast infection during their lifetime; 90 percent of these infections are caused by Candida albicans. Further estimates indicate that 5 percent of women with vulvovaginal candidiasis may develop RVVC, which is defined as four or more episodes of vulvovaginal candidiasis in the previous year.6 Species such as Candida glabrata, Candida parapsilosis and Saccharomyces cerevisiae are responsible for up to 33 percent of recurrent infections.7 As will be discussed later, there may be inherent differences between C. albicans and non–C. albicans infections; therefore, obtaining a positive fungal culture, which includes identifying the infecting organism, is an essential first step in the management of RVVC.
Three primary theories have been proposed to explain why some women develop RVVC. The intestinal reservoir theory suggests that the recurrences are a result of persistence of the organism in the gastrointestinal tract and later reinfection of the vagina. This theory is based on uncontrolled data8 from the late 1970s in which a concordance of almost 100 percent was observed between rectal and vaginal cultures in women with RVVC. However, subsequent studies9 suggest that women with RVVC harbor yeast species in the gastrointestinal tract as often as control subjects. Furthermore, in women who experienced a recurrence after taking prolonged courses of systemic ketoconazole for RVVC and who experienced a recurrence following cessation of therapy, the recurrence often occurred in the presence of negative rectal cultures for yeast.10 The results from these two studies make it less likely that the intestinal reservoir theory satisfactorily explains the cause of RVVC.
The sexual transmission theory views the partner as the source of the reinfection. Indeed, at least 20 percent of the partners of women with RVVC harbor the same yeast species in their mouth, fingers or genital area.10 However, in the majority of cases, the partner is culture-negative and cannot be implicated as the source of reinfection. Furthermore, at least one longitudinal study11 of couples in which the woman had RVVC indicates that the higher colonization rate in the man is more a reflection of his exposure to the chronically infected woman than an indication that the transmission occurred in the other direction. On a practical level, treatment of the partner seems to have no effect on the woman's risk of recurrence12 and is not recommended by most experts.6
The vaginal relapse theory maintains that, even after treatment, some women remain colonized with small numbers of yeast. Given the proper conditions, the yeast increase in number and cause a new clinical episode of vulvovaginal candidiasis. Support for this theory includes longitudinal studies13 that document persistence of the same strain of C. albicans causing repeated infections in the same women over several years of observation and after repeated treatment courses. According to this view, repeated episodes are not the result of reinfections, but rather caused by host factors. Although obvious exogenous factors such as diabetes, use of antibiotics and systemic corticosteroids, and infection with human immunodeficiency virus may play a role in RVVC, no obvious explanation exists for most recurrences. Much research is currently focused on possible abnormalities of the local vaginal immune response to yeast and their role in laying the groundwork for the patient's next infection.14
When treating women with RVVC, controlling any underlying medical conditions may be helpful. Although dietary changes (i.e., a yeast-free diet) have been frequently advocated as a treatment for RVVC, no studies have documented any efficacy to this approach. When the infection is caused by C. albicans, RVVC is best managed using an initial 14-day course of oral azole therapy to induce clinical remission and a negative fungal culture, followed by a six-month maintenance regimen.6 Maintenance regimens include ketoconazole (Nizoral), 100 mg daily; itraconazole (Sporanox), 100 mg daily; and fluconazole (Diflucan), 100 to 200 mg weekly.
An alternative topical maintenance regimen consists of clotrimazole vaginal suppositories (Gyne-Lotrimin), 500 mg weekly. An examination and reculture of the patient after the initial two-week regimen and then at the three- and six-month points in the maintenance regimen will ensure that the antimycotic therapy is effective and that the patient's symptoms have resolved. While on maintenance therapy, at least 90 percent of patients will not experience a recurrence. Furthermore, particularly with oral regimens, at least some of the protective effects persist after discontinuation of therapy.15 Treatment should be individualized for patients who experience a recurrence following completion of a maintenance regimen, but the option of restarting a maintenance regimen if the recurrences become frequent should be considered.
In cases of RVVC secondary to C. albicans, resistance to antifungal therapy seems rare in that the vast majority of patients will, at a minimum, do well while on antifungal maintenance regimens. However, for infections caused by non–C. albicans species, particularly those due to C. glabrata, clinically evident resistance seems more common.7,16 In patients who failed standard azole therapy, boric acid vaginal suppositories (600 mg daily for 14 days)16 and topical flucytosine (Ancobon) cream have been used successfully.17 Treating patients who continue to have symptoms while on therapy or who experience a recurrence shortly after completing therapy is a particularly difficult problem. Information about the treatment of these patients remains limited to anecdotal and unpublished data. However, using combination therapy and extending the duration of treatment seem to be logical approaches to this difficult clinical scenario, particularly if frequent reculturing is performed to assess the mycologic response.
Final Comment
Chronic vulvovaginal symptoms are relatively common and may be difficult for both the patient and the physician. However, by placing an emphasis on obtaining an accurate diagnosis, the cause of the patient's symptoms can often be determined. In women who fail to respond to treatment, it is important to reestablish the diagnosis to make sure that the cause of the symptoms remains the same.