
Am Fam Physician. 2002;65(9):1823-1831
A more recent article on acute respiratory distress syndrome is available.
Acute respiratory distress syndrome is the clinical manifestation of severe, acute lung injury. It is characterized by the acute onset of diffuse, bilateral pulmonary infiltrates secondary to noncardiogenic pulmonary edema, refractory hypoxia, and decreased lung compliance. Acute respiratory distress syndrome occurs most frequently in the setting of sepsis, aspiration of gastric contents, trauma, or multiple transfusions. Its complex pathophysiology involves an inciting local or systemic event that initiates pulmonary endothelial and epithelial damage and subsequent increased permeability. Tachypnea, hypoxia, and respiratory alkalosis are typical early clinical manifestations, and they are usually followed by the appearance of diffuse pulmonary infiltrates and respiratory failure within 48 hours. Early identification and treatment of the underlying disorder, along with aggressive supportive care, are essential. Experimental therapies, including those using nitric oxide and surfactant, have not been shown to improve mortality in patients with ARDS, but new therapeutic approaches such as low-volume ventilation have been shown to decrease mortality. Many patients who survive ARDS have permanent, mild to moderate impairment of lung function. Quality of life after hospitalization with ARDS may be poorer than that in similar patients without ARDS.
Acute respiratory distress syndrome (ARDS) is characterized by acute lung injury, noncardiogenic pulmonary edema, and severe hypoxia. A common condition with a complex pathophysiology, it frequently occurs in critically ill medical and surgical patients. Since ARDS was first described in 1967,1 great advances have been made in understanding its pathophysiology.
Numerous previous trials of new pharmacologic and ventilatory modalities have failed to demonstrate improvement in mortality rates. However, recent trials using a new “lung protective” approach to mechanical ventilation resulted in significant reductions in mortality rates.2,3 Data from these studies represent an encouraging breakthrough in the management of ARDS.
Many family physicians care for patients with ARDS, and many patients who survive ARDS receive their post-intensive care unit (ICU) treatment from family physicians. Therefore, a comprehensive understanding of this syndrome is essential.
Definition
The most widely used definition of ARDS was published in an American-European Consensus Conference statement in 1994.4 It described ARDS as a syndrome of inflammation and increased permeability associated with a constellation of clinical, radiologic, and physiologic abnormalities unexplained by elevations in left atrial or pulmonary capillary pressure. Defining diagnostic criteria are outlined in Table 1. These criteria are easy to identify clinically and take into account the severity of lung injury.5 Patients with a partial pressure of arterial oxygen (Pao2) to percentage of inspired oxygen (Fio2) ratio of 300 or less are considered to have “acute lung injury,” whereas patients with more severe hypoxia (defined as a measured partial pressure of arterial oxygen over the fraction of inspired oxygen [Pao2/Fio2] ratio of less than 200) are classified as having ARDS.
| Identifiable associated condition |
| Acute onset |
| Pulmonary artery wedge pressure ≤ 18 mm Hg or absence of clinical evidence of left atrial hypertension |
| Bilateral infiltrates on chest radiography |
| Acute lung injury is present if Pao2/Fio2 ratio is ≤ 300* |
| Acute respiratory distress syndrome is present if Pao2/Fio2 ratio ≤ 200 |
Epidemiology
INCIDENCE
In 1972, a National Institutes of Health panel estimated the incidence of ARDS to be approximately 75 per 100,000 population per year.6 However, a general agreement now finds that this is an overestimation. In prospective studies, the incidence of ARDS has been reported to range from 1.5 to 8.3 cases per 100,000 population per year.7,8 These reports have been criticized for excluding patients with a Pao2/Fio2 ratio greater than 150. Therefore, the incidence may be higher than these figures suggest.
RISK FACTORS
Clinical conditions associated with ARDS have been identified (Table 2).5 These conditions are divided into two categories: those that cause direct lung injury and those that cause indirect lung injury by activating a systemic inflammatory response. Of the various risk factors, sepsis is associated with the highest risk of progression to ARDS. Aspiration of gastric contents, multiple trauma, multiple blood transfusions, diffuse pneumonia, and disseminated intravascular coagulation are also associated with a high risk of developing ARDS. Certain preexisting conditions, such as chronic lung disease and chronic alcoholism, significantly increase the risk of developing ARDS.9,10 ARDS appears to be more common in patients over 65 years of age in patient populations with similar risk factors.9 However, gender seems to have no effect on the likelihood of developing the syndrome.9
Histopathologic Changes
Diffuse alveolar damage is the descriptive term for the histopathologic findings encountered in acute lung injury. Diffuse alveolar damage is divided into exudative (Figure 1) and fibroproliferative phases. Although each phase has its own histologic characteristics, there is significant overlap between the two stages (Figure 2), which are clinically indistinguishable. The extent of fibrosis is highly variable among patients. It may be absent in some, while severe fibrosis with honeycombing may develop in others.
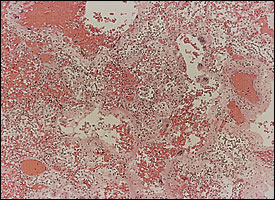
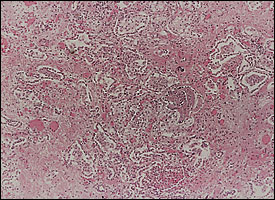
Pathogenesis
The exact sequence of events in ARDS is unknown. In response to direct lung injury or a systemic insult such as endotoxin, an increase in pulmonary or circulatory pro-inflammatory cytokines occurs. There may also be perturbations in the pro- and anti-inflammatory cytokine balance.11 Neutrophils activated by cytokines adhere to the pulmonary capillary endothelial surface and migrate into the interstitial and alveolar spaces. Activated neutrophils secrete cytokines, such as tumor necrosis factor-alpha and interleukins, which increase the inflammatory response. Neutrophils also produce oxygen radicals and proteases that can injure the capillary endothelium and alveolar epithelium.5 Neutrophil arachidonic acid metabolites can cause alterations in pulmonary vascular auto-regulation and vascular permeability.12 Epithelial and endothelial damage, in turn, leads to increased permeability and the subsequent influx of protein-rich fluid into the alveolar space. In addition to these structural changes, there is evidence of impaired fibrinolysis in ARDS that leads to capillary thrombosis and microinfarction.
However, the principal role of the neutrophil has been questioned. For example, ARDS can develop in neutropenic patients. A variety of inflammatory mediations are also produced by alveolar macrophages.
Understanding of the fibroproliferative process is limited. Some patients achieve complete resolution of lung injury before progressing into the fibroproliferative stage, whereas others progress directly to develop fibrosis. The extent of fibrosis may be determined by the severity of the initial injury, ongoing or repetitious lung injury, toxic oxygen effects, and ventilator-associated lung injury.
Clinical Findings
PRESENTATION
A progression of clinical findings is present in patients with ARDS. Tachypnea, tachycardia, and respiratory alkalosis usually develop within the first 12 to 24 hours after the inciting event and may precede the appearance of infiltrates on a chest radiograph. The inflammatory process and alveolar flooding lead to severe ventilation-perfusion mismatch and intrapulmonary shunt, which are manifested clinically as severe hypoxia with a decrease in the Pao2/Fio2 ratio. Generally, a marked reduction in lung compliance occurs, which increases the work of breathing. The physiologic dead space increases in ARDS and, to maintain a normal or near-normal partial pressure of carbon dioxide (PCO2), patients must increase their minute ventilation.
The severe hypoxia, increased dead space, and decreased lung compliance all contribute to the development of respiratory failure. Most patients evolve diffuse alveolar infiltrates and progress to respiratory failure within 48 hours of the onset of symptoms.
LUNG IMAGING
During the exudative phase, chest radiographs reveal a progression from diffuse interstitial infiltrates to diffuse, fluffy, alveolar opacities (Figure 3). Although ARDS cannot reliably be distinguished from cardiogenic pulmonary edema on radiologic grounds, patients with ARDS often lack cardiomegaly, pleural effusions, and vascular redistribution. Reticular opacities may ensue, suggesting the development of interstitial fibrosis.
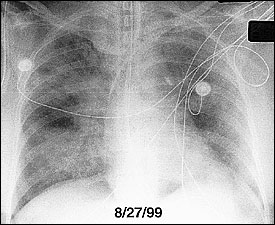
Computed tomographic (CT) scans of the chest have led to increased understanding of the structural abnormalities occurring at different stages of ARDS.13 Bilateral alveolar opacities are seen during the acute phase of ARDS and are more pronounced in the dependent, posterior lung zones. The anterior lung fields are relatively spared, highlighting the heterogeneous distribution of inflammation in the lungs. CT scans can reveal marked improvement in the aeration of the dependent (posterior) lung regions with the application of positive end-expiratory pressure (PEEP) (Figure 4).14 Bilateral reticular opacities, reduced lung volumes and, occasionally, large bullae are CT findings during the fibroproliferative stage.
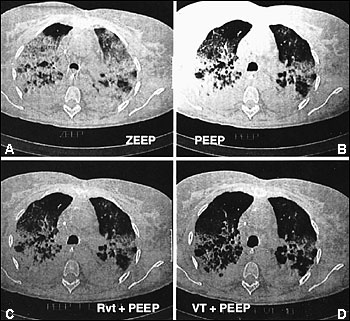
Management
NONVENTILATORY SUPPORTIVE CARE
The general principles of nonventilatory supportive care are outlined in Table 3. A reported decline in mortality rate in patients with ARDS has been attributed to advances in supportive care.15 An expeditious search for the underlying cause of ARDS and aggressive early treatment are key components in mortality reduction.
| Nonventilatory supportive care Identify early and aggressively treat underlying cause. Minimize potential complications such as nosocomial infections, gastrointestinal bleeding, and thromboembolism. Initiate appropriate nutrition. Maintain appropriate level of sedation to facilitate patient-ventilator synchrony and limit patient discomfort. Limit excessive and prolonged sedation and use of paralytic agents. | Ventilation management Set initial tidal volume at 6 mL per kg (based on predicted body weight*). Aim to maintain Pplat at < 30 mm Hg (may require subsequent adjustments of tidal volume). Ensure adequate oxygenation: Pao2 of 55 to 80 mm Hg or Spo2 of 88 to 95%. Ensure adequate carbon dioxide removal. Minimize oxygen toxicity by maintaining Fio2 at < 60% Ensure maximal recruitment of alveoli through appropriate levels of PEEP. |
Fluid management is controversial. Results from two studies suggest that limiting fluid retention in patients with ARDS leads to less pulmonary edema and decreased mortality.16,17 However, most patients with ARDS die from multiple organ failure, and some investigators and clinicians have expressed concern that reducing intravascular volume may impair oxygen delivery to the tissues and increase the risk of developing multiple organ failure.
Another component of supportive care is attempting to minimize the development of complications such as nosocomial infection, gastrointestinal bleeding, and thromboembolism. Stress ulcer prophylaxis is indicated in all patients with ARDS, although there is some debate about the preferred agent. The risk of nosocomial infection can be reduced with careful hand washing and the use of full-barrier precautions during the insertion of intravascular devices. Some form of deep venous thrombosis prophylaxis is indicated in most patients with ARDS. All patients with ARDS require nutritional support and, in most instances, enteral administration is preferable.
PHARMACOLOGIC TREATMENT
A myriad of trials of experimental agents for the treatment of ARDS have been undertaken. A detailed review of the trials can be found in a recent American-European ARDS consensus statement.18 Inhaled nitric oxide, a pulmonary vasodilator, is distributed to regions of the lung that are best ventilated and, by increasing the blood flow to those regions, improves ventilation-perfusion matching. When given at concentrations of up to 20 parts per million, nitric oxide has been shown to improve oxygenation.19 However, two large randomized trials failed to show a significant reduction in the duration of mechanical ventilation or mortality.20,21
Corticosteroids and exogenous surfactant treatments have been evaluated because inflammation and alterations in surfactant function are features of ARDS. Methylprednisolone administered before the onset of ARDS or during its early course does not decrease mortality.22,23 However, results from a recent, small randomized trial showed significant improvement in mortality when corticosteroid therapy was initiated seven days after the diagnosis was made.24 Although these results are promising, they are not definitive. A larger randomized trial of methylprednisolone administered during the fibrosing stage of ARDS is currently underway.
Administration of surfactant in neonates with infant respiratory distress syndrome has led to improved survival rates. In adults, however, use of surfactant did not improve oxygenation, lessen the duration of mechanical ventilation, or decrease mortality.25 Improved preparations and modes of administration via bronchoscopy are currently being investigated.
In most patients with ARDS, sedation is necessary to facilitate patient-ventilator synchrony and reduce oxygen demand. Occasionally, neuromuscular blocking agents are required to provide effective ventilation but, given the short- and long-term effects of these agents, their use should be limited, and the depth of paralysis should be carefully monitored.
VENTILATORY SUPPORT
The general principles of ventilatory management are outlined in Table 3. In the past, many clinicians delivered relatively large tidal volumes and hesitated to use PEEP in patients with ARDS. However, an increased understanding of ventilator-associated lung injury and the results of recent studies have cast doubt on that strategy. In the animal model, overdistention causes acute lung injury and may exacerbate preexisting acute lung injury. Similarly, the cyclic opening and closing of small airways and alveoli may cause injury because of “shear forces.” Given the heterogeneous distribution of lung injury in ARDS, the use of traditional tidal volumes of 10 to 15 mL per kg may overexpand the normal lung areas and cause further lung injury.
Results from a recent, large, multicenter randomized trial have shown that, compared with a traditional approach to mechanical ventilation, a strategy aimed at delivering lower tidal volumes and limiting plateau pressure resulted in a 22 percent reduction in mortality2(Table 3). A smaller study combining low tidal volume ventilation with high PEEP also showed decreased mortality.3 The use of higher levels of PEEP presumably prevents the end-tidal collapse of alveolar units, which in turn reduces repetitive opening and closing and stress-induced damage to alveoli.
Other approaches, such as partial liquid ventilation and high-frequency oscillatory ventilation, have been promising. Prone positioning has been investigated. Placing patients in the prone position can lead to improvements in oxygenation, possibly by reducing edema and atelectasis in the posterior dependent lung zones, thereby improving ventilation-perfusion mismatch.26 Despite the reports of short-term improvement in oxygenation, a recent randomized, prospective trial failed to show a decrease in mortality with prone ventilation.27
Complications, Recovery, and Outcomes
In addition to the underlying disorders, morbidity and mortality associated with ARDS may be influenced by the many complications associated with management of the syndrome (Table 4). Most patients who die from ARDS do so within the first two weeks following diagnosis. Those who survive often have a prolonged course in the ICU. Therefore, long-term supportive care, including tracheostomy, may be necessary.
| Complication | Preventive measures |
|---|---|
| Neck/thoracic | |
| Tracheal stenosis, vocal cord dysfunction | Identify appropriate time for tracheostomy |
| Ventilator-associated pneumonia | Head elevation, suctioning, expeditious weaning |
| Gastrointestinal | |
| Stress-related gastrointestinal hemorrhage | Use of stress ulcer prophylaxis |
| Barotrauma | |
| Pneumothorax, pneumomediastinum, pneumoperitoneum, air embolism | Limit airway and/or plateau pressures |
| Cardiac/hemodynamic | |
| Hypotension | Limit excessive diuresis; limit excessive use of PEEP |
| Vascular | |
| Mechanical damage from central line placement | Careful attention to appropriate central line placement technique |
| Other | |
| Excessive sedation | Titrate sedation according to sedation assessment scales |
| Excessive paralysis | Continuous monitoring of level of paralysis with train of four stimulation |
Neuropsychiatric and physical functi
| Function | Within two weeks of extubation* | Six months following extubation* |
|---|---|---|
| DLco | 71 | 26 |
| TLC | 76 | 46 |
| FVC | 95 | 55 |
| FEV1 | 5 | 25 |
DLco = carbon monoxide diffusing capacity; TLC = total lung capacity; FVC = forced vital capacity; FEV1 = forced expiratory volume in one second.
*—Percentage of patients.
Information from reference28 .
ons are impaired in patients who survive ARDS, but the in-hospital factors that lead to poorer quality of life have not been identified. A recent study comparing trauma and sepsis patients with and without ARDS found a significant reduction in physical function, general health, and mental health in the patients with ARDS.29 Therefore, ARDS may confer an additional burden of morbidity independent of other co-morbidities.