
A more recent article on heart failure is available.
Am Fam Physician. 2004;69(11):2609-2617
Diastolic heart failure, a major cause of morbidity and mortality, is defined as symptoms of heart failure in a patient with preserved left ventricular function. It is characterized by a stiff left ventricle with decreased compliance and impaired relaxation, which leads to increased end diastolic pressure. Signs and symptoms are similar to those of heart failure with systolic dysfunction. The diagnosis of diastolic heart failure is best made with Doppler echocardiography. Based on current knowledge, pharmacologic treatment of diastolic heart failure should focus on normalizing blood pressure, promoting regression of left ventricular hypertrophy, avoiding tachycardia, treating symptoms of congestion, and maintaining normal atrial contraction when possible. Diuretic therapy is the mainstay of treatment for preventing pulmonary congestion, while beta blockers appear to be useful in preventing tachycardia and thereby prolonging left ventricular diastolic filling time. Angiotensin-converting enzyme inhibitors and angiotensin-receptor blockers may be beneficial in patients with diastolic dysfunction, especially those with hypertension. Evidence from adequately powered randomized controlled trials, however, is not available yet. The outcomes of ongoing clinical trials may provide much-needed information to move from intuitive treatment to therapy based on evidence that matters: decreased morbidity and mortality and improved quality of life.
Heart failure affects approximately 4.8 million persons in the United States, with about 500,000 new cases diagnosed each year.1,2 It is the leading cause of hospitalization in patients older than 65 years.3 In spite of significant advances in the treatment of heart failure, mortality rates remain high: 30 to 40 percent of patients with advanced disease and 5 to 10 percent of patients with mild symptoms die within five to 10 years.4
Heart failure can arise from any condition that compromises the contractility of the heart (systolic heart failure) or that interferes with the heart's ability to relax (diastolic heart failure). Hospital- and community-based reports indicate that about one fourth to one half of patients with heart failure have normal left ventricular systolic function.5,6 Observational studies5–7 indicate that diastolic heart failure is more common in women and elderly persons. Although patients with diastolic heart failure have a lower annual mortality rate than patients with systolic heart failure, they have a higher rate than the general population. They also have hospitalization rates similar to those of patients with systolic heart failure. These observations emphasize diastolic heart failure as an important contributor to morbidity, mortality, and health care costs, and highlight the need for further research and clinical trials examining this condition.
Pathophysiology
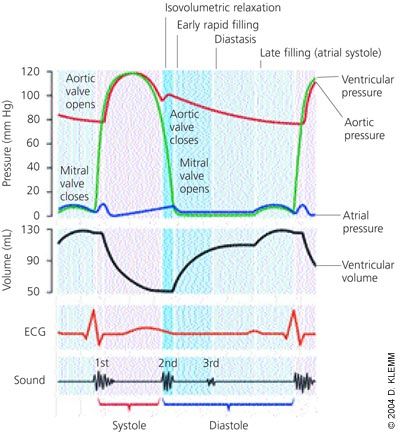
Diastole is the process by which the heart returns to its relaxed state; it is also the time for cardiac perfusion. During diastole, drastic changes in cardiac pressure-volume relationships occur. The relaxation process has four identifiable phases: isovolumetric relaxation from the time of aortic valve closure to mitral valve opening; early rapid filling after mitral valve opening; diastasis, a period of low flow during mid-diastole; and late filling of the ventricles from atrial contraction (Figure 1).
In patients with isolated diastolic heart failure, the heart often is able to meet the body's metabolic needs, but at higher diastolic pressures. The left ventricle is stiff, with decreased compliance and impaired relaxation. Transmission of the higher end-diastolic left ventricular pressure to the pulmonary circulation may lead to pulmonary congestion, dyspnea, and other symptoms of heart failure.8,9
Diastole is a complex process that is affected by a number of factors, including ischemia, heart rate, velocity of relaxation, cardiac compliance (i.e., elastic recoil and stiffness), hypertrophy, and segmental wall coordination of the heart muscle.
HYPERTENSION
Chronic hypertension is the most common cause of diastolic dysfunction and failure. It leads to left ventricular hypertrophy and increased connective tissue content, both of which decrease cardiac compliance.10 The hypertrophied ventricle has a steeper diastolic pressure-volume relationship; therefore, a small increase in left ventricular end-diastolic volume (which can occur with exercise, for example) causes a marked increase in left ventricular end-diastolic pressure.
ISCHEMIA
Relaxation of the ventricles involves the active transport of calcium ions into the sarcoplasmic reticulum, which allows the dissociation of myosinactin crossbridges. Hypoxia inhibits the dissociation process by altering the balance of the adenosine triphosphate–to–adenosine diphosphate ratio, which may contribute to diastolic dysfunction.11
HEART RATE
The heart rate determines the time that is available for diastolic filling, coronary perfusion, and ventricular relaxation. Tachycardia adversely affects diastolic function by several mechanisms: it decreases left ventricular filling and coronary perfusion times, increases myocardial oxygen consumption, and causes incomplete relaxation because the stiff heart cannot increase its velocity of relaxation as heart rate increases. Patients with diastolic dysfunction do not tolerate tachycardia or exercise well.
ATRIAL FIBRILLATION
Patients with heart failure are at increased risk for atrial fibrillation.12 As the ventricle stiffens and develops higher end-diastolic pressures, the atria are distended and stressed; this situation often results in atrial fibrillation. The loss of atrial contraction worsens the symptoms of heart failure, because patients with diastolic dysfunction often are dependent on atrial filling of the left ventricle (“atrial kick”). Atrial fibrillation also can worsen symptoms if the ventricular rate is uncontrolled.
VENTRICULAR LOAD
At the end of normal systole, a small residual volume of blood remains in the left ventricle. If this residual volume increases, it interferes with the normal elastic recoil of the heart, the relaxation of the heart, and the development of a negative pressure gradient between the ventricle and atria. As a result, rapid early diastolic filling is impaired.
AGING
Diastolic dysfunction is more common in elderly persons, partly because of increased collagen cross-linking, increased smooth muscle content, and loss of elastic fibers.13,14 These changes tend to decrease ventricular compliance, making patients with diastolic dysfunction more susceptible to the adverse effects of hypertension, tachycardia, and atrial fibrillation.
Diagnosis
The signs and symptoms of heart failure are nonspecific (dyspnea, exercise intolerance, fatigue, weakness) and often can be attributed to other conditions, such as pulmonary disease, anemia, hypothyroidism, depression, and obesity. Furthermore, it is difficult to distinguish diastolic from systolic heart failure based on physical findings or symptoms (Table 115–17).15,18–20 Systolic heart failure is defined as a left ventricular ejection fraction of less than 45 percent, but diagnostic criteria for diastolic dysfunction are still controversial.
Cardiac catheterization remains the gold standard for demonstrating impaired relaxation and filling, because it provides direct measurement of ventricular diastolic pressure. However, the balance of benefit, harm, and cost argue against its routine use in diagnosing diastolic dysfunction.
Doppler echocardiography has assumed the primary role in the noninvasive assessment of cardiac diastolic function and is used to confirm the diagnosis of diastolic heart failure. For example, echocardiographic measurement of tau, the time constant of left ventricular pressure decay during isovolumetric relaxation, can be performed to assess left ventricular stiffness.
More importantly, Doppler echocardiogra phy is used to evaluate the characteristics of diastolic trans–mitral-valve blood flow. The peak velocities of blood flow during early diastolic filling (E wave) and atrial contraction (A wave) are measured, and the ratio is calculated. Under normal conditions, the early-filling E-wave velocity is greater than the A-wave velocity, and the E-to-A-wave ratio is about 1.5 (Figure 2). In early diastolic dysfunction, this relationship reverses, because the stiffer heart relaxes more slowly, and the E-to-A-wave ratio drops below 1.0 (Figure 3). As diastolic function worsens and left ventricular diastolic pressure rises, left ventricular diastolic filling occurs primarily during early diastole, because the left ventricular pressure at end-diastole is so high that atrial contraction contributes less to left ventricular filling than normal. At this point, the E-to-A-wave ratio rises, often to greater than 2.0 (Figure 4). This so-called “restrictive pattern” confers a poor prognosis.21 Recent studies also have shown that Doppler evaluation of myocardial velocities during ventricular relaxation predict elevated filling pressure.
The E- and A-wave velocities are affected by blood volume and mitral valve anatomy and function. Furthermore, these wave velocities are less useful in the setting of atrial fibrillation. Despite these limitations, Doppler echocardiography provides essential information about the anatomy and function of the heart, chamber size, hypertrophy, valvular function, regional wall abnormalities, and chamber pressures. It also allows the physician to identify and rule out other potential causes of the patient's symptoms, such as valvular lesions, pericardial disease, and pulmonary hypertension.

The serum level of B-type natriuretic peptide is an accurate tool for establishing the diagnosis of heart failure in patients with dyspnea, but the test cannot distinguish diastolic from systolic heart failure.22,23 A B-type natriuretic peptide level greater than 100 pg per mL was found to be 95 percent sensitive but only 14 percent specific for detecting systolic heart failure in a mixed group of patients with systolic or diastolic heart failure.24

In an attempt to establish diagnostic criteria, the European Study Group on Diastolic Heart Failure25 proposed three obligatory conditions for the diagnosis of diastolic heart failure: the presence of signs and symptoms of heart failure; the presence of normal or mildly abnormal left ventricular systolic function (ejection fraction of greater than 45 percent); and evidence of abnormal left ventricular relaxation, filling, diastolic distensibility, or diastolic stiffness. The criteria have been criticized, however, because symptoms of heart failure are nonspecific, significant variability exists in eliciting and reporting symptoms of heart failure, and it is impractical to obtain evidence of abnormal relaxation in daily clinical practice.
One investigative team26 proposed classifications of definite, probable, and possible diastolic heart failure, depending on the presence of three stratified conditions: definite evidence of heart failure; objective evidence of normal left ventricular systolic function measured within 72 hours of the heart failure event; and objective evidence of left ventricular diastolic dysfunction by cardiac catheterization. If all three conditions are present, the diagnosis of diastolic heart failure is definite. If the first two conditions are present, the diagnosis is probable. If only the first condition is present or only partial evidence of the second condition is present, the diagnosis of diastolic heart failure is considered possible. A recent prospective study of 63 patients with symptoms of heart failure based on the Framingham criteria27 and a normal ejection fraction found that more than 90 percent of the patients had abnormal diastolic function on evaluation with both Doppler echocardiography and cardiac catheterization. The results of this study call into question the need for objective measurement of diastolic dysfunction, but confirmation is required before this approach is adopted more widely.

Treatment
The treatment of diastolic heart failure is less well defined than the treatment of systolic heart failure. Current recommendations are based on disease-oriented evidence, including pathophysiology, extrapolation of knowledge about other aspects of cardiovascular disease, data from small studies, and expert opinion. None of the treatment recommendations have been validated by randomized controlled trials (RCTs). Evidence-based guidelines from the American College of Cardiology/American Heart Association (ACC/AHA)28 and the Institute for Clinical Systems Improvement (ICSI)29 provide guidance until RCTs can be completed.
NONPHARMACOLOGIC INTERVENTIONS
Lifestyle modifications are recommended to reduce the risk of all forms of cardiovascular disease. Measures include weight loss, smoking cessation, dietary changes, and exercise. Identification and treatment of comorbid conditions, such as high blood pressure, diabetes, and hypercholesterolemia, are important in reducing the risk of subsequent heart failure.28,29
PHARMACOLOGIC THERAPY
Pharmacologic treatment of diastolic heart failure is directed at normalizing blood pressure, promoting regression of left ventricular hypertrophy, preventing tachycardia, treating symptoms of congestion, and maintaining atrial contraction.28–30 The basis for drug therapy remains empiric, and the recommendations discussed in this section come from the ACC/AHA guidelines and the ICSI evidence-based practice guideline.28–30
Treatment with diuretics and vasodilators often is necessary to reduce pulmonary congestion. However, caution is required to avoid excessive diuresis, which can decrease preload and stroke volume. Patients with diastolic dysfunction are highly sensitive to volume changes and preload.
The potential benefits of beta blockers stem from their ability to decrease heart rate, increase diastolic filling time, decrease oxygen consumption, lower blood pressure, and cause regression of left ventricular hypertrophy. The Study of the Effects of Nebivolol Intervention on Outcomes and Rehospitalisation in Seniors with Heart Failure31 is evaluating the effect of the beta blocker nebivolol in the treatment of elderly patients with diastolic heart failure.
The multiple benefits of angiotensin-converting enzyme (ACE) inhibitors in the treatment of cardiovascular disease make them highly promising therapeutic agents. ACE inhibitors cause regression of left ventricular hypertrophy, decrease blood pressure, and prevent or modify cardiac remodeling; these actions provide strong theoretic support for the use of these agents in patients with diastolic dysfunction. So far, there have been few studies of ACE inhibitors in patients with diastolic dysfunction.32 The Perindopril for Elderly People with Chronic Heart Failure study33 is the largest ongoing trial of the benefits of perindopril in patients with diastolic failure.
| Key clinical recommendations for patients with diastolic dysfunction | Strength of recommendation | References |
|---|---|---|
| Treat blood pressure to achieve a goal of less than 130/85 mm Hg. | A | 28,29 |
| For patients with concomitant atrial fibrillation, control ventricular rate or restore sinus rhythm. | C | 28 |
| Coronary revascularization is recommended in patients with coronary artery disease in whom symptomatic or demonstrable myocardial ischemia is judged to have an adverse effect on diastolic function. | C | 28 |
| Use diuretics to control pulmonary congestion and peripheral edema. | C | 28,29 |
| Use digitalis to minimize symptoms of heart failure. | C | 28 |
| Use beta blockers for rate control, especially in patients with atrial fibrillation or symptoms of heart failure. Start with a low dosage; higher dosages can be used in patients with diastolic dysfunction than in patients with systolic dysfunction. | C | 28,29 |
| Use angiotensin-converting enzyme inhibitors to control symptoms of heart failure. | C | 28,29 |
One arm of the Candesartan in Heart Failure-Assessment of Reduction of Mortality and Morbidity trial34 studied the effect of candesartan in patients with preserved systolic function. After an average of 36.6 months follow-up, the study found no difference in cardiovascular mortality but a small decrease in hospitalization for worsening heart failure among patients taking candesartan as compared with placebo. In 1999, a small trial showed that losartan improved exercise tolerance in patients with diastolic dysfunction and a hypertensive response to exercise.35 The Irbesartan in Heart Failure with Preserved Systolic Function study is currently evaluating the utility of angiotensin-receptor blockers in patients with diastolic heart failure.
In theory, the use of calcium channel blockers may be beneficial, because these agents decrease blood pressure, decrease oxygen demand, and dilate coronary arteries. However, data are lacking on patient-oriented outcomes such as morbidity and mortality. Calcium channel blockers should be used with caution in patients with coexisting systolic and diastolic dysfunction. The long-acting dihydropyridine class of calcium channel blockers is safe for use in patients with systolic heart failure, but nondihydropyridine agents should be avoided.