
A more recent article on community-acquired pneumonia in adults is available.
Am Fam Physician. 2011;83(11):1299-1306
Author disclosure: Nothing to disclose.
Community-acquired pneumonia is diagnosed by clinical features (e.g., cough, fever, pleuritic chest pain) and by lung imaging, usually an infiltrate seen on chest radiography. Initial evaluation should determine the need for hospitalization versus outpatient management using validated mortality or severity prediction scores. Selected diagnostic laboratory testing, such as sputum and blood cultures, is indicated for inpatients with severe illness but is rarely useful for outpatients. Initial outpatient therapy should include a macrolide or doxycycline. For outpatients with comorbidities or who have used antibiotics within the previous three months, a respiratory fluoroquinolone (levofloxacin, gemifloxacin, or moxifloxacin), or an oral beta-lactam antibiotic plus a macrolide should be used. Inpatients not admitted to an intensive care unit should receive a respiratory fluoroquinolone, or a beta-lactam antibiotic plus a macrolide. Patients with severe community-acquired pneumonia or who are admitted to the intensive care unit should be treated with a beta-lactam antibiotic, plus azithromycin or a respiratory fluoroquinolone. Those with risk factors for Pseudomonas should be treated with a beta-lactam antibiotic (piperacillin/tazobactam, imipenem/cilastatin, meropenem, doripenem, or cefepime), plus an aminoglycoside and azithromycin or an antipseudomonal fluoroquinolone (levofloxacin or ciprofloxacin). Those with risk factors for methicillin-resistant Staphylococcus aureus should be given vancomycin or linezolid. Hospitalized patients may be switched from intravenous to oral antibiotics after they have clinical improvement and are able to tolerate oral medications, typically in the first three days. Adherence to the Infectious Diseases Society of America/American Thoracic Society guidelines for the management of community-acquired pneumonia has been shown to improve patient outcomes. Physicians should promote pneumococcal and influenza vaccination as a means to prevent community-acquired pneumonia and pneumococcal bacteremia.
Community-acquired pneumonia (CAP) is a significant cause of morbidity and mortality in adults. CAP is defined as an infection of the lung parenchyma that is not acquired in a hospital, long-term care facility, or other recent contact with the health care system. Table 1 includes common etiologies of CAP.1–3 This article discusses the important studies and guidelines for CAP that have been published since the topic was last reviewed in American Family Physician.4
| Clinical recommendations | Evidence rating | References |
|---|---|---|
| In patients with clinically suspected CAP, chest radiography should be obtained to confirm the diagnosis. | C | 12 |
| Evaluation for specific pathogens that would alter standard empiric therapy should be performed when the presence of such pathogens is suspected on the basis of clinical and epidemiologic clues; this testing usually is not required in outpatients. | C | 12 |
| Mortality and severity prediction scores should be used to determine inpatient versus outpatient care for patients with CAP. | A | 22–24 |
| All patients with CAP who are admitted to the intensive care unit should be treated with dual therapy. | A | 28 |
| Prevention of CAP should focus on universal influenza vaccination and pneumococcal vaccination for patients at high risk of pneumococcal disease. | B | 12, 35–37 |

| Etiology | Frequency (median percentage) | Etiology | Frequency (median percentage) | Etiology | Frequency (median percentage) |
|---|---|---|---|---|---|
| Outpatients | Inpatients not admitted to ICU | Inpatients admitted to ICU | |||
| Mycoplasma pneumoniae | 16 | S. pneumoniae | 25 | S. pneumoniae | 17 |
| Respiratory viruses | 15 | Respiratory viruses | 10 | Legionella species | 10 |
| Streptococcal pneumoniae | 14 | M. pneumoniae | 6 | Gram-negative bacilli | 5 |
| Chlamydophila pneumoniae | 12 | H. influenzae | 5 | Staphylococcus aureus | 5 |
| Legionella species | 2 | C. pneumoniae | 3 | Respiratory viruses | 4 |
| Haemophilus influenzae | 1 | Legionella species | 3 | H. influenzae | 3 |
| Unknown | 44 | Unknown | 37 | Unknown | 41 |
Epidemiology
Pneumonia and influenza combined is the eighth leading cause of death in the United States and the most common cause of infection-related mortality.5 In 2007, about 52,700 persons died from the conditions.5 The overall annual incidence of CAP ranges from five to 11 per 1,000 persons, with more cases occurring in the winter months.1 In 2006, there were approximately 4.2 million ambulatory care visits for CAP in the United States, with Streptococcus pneumoniae as the most commonly identified pathogen.6 The estimated annual economic burden of CAP in the United States exceeds $17 billion.6
Diagnosis
DIFFERENTIAL DIAGNOSIS
Many microbiologic pathogens can cause CAP. Pneumonia traditionally has been classified as typical, usually caused by S. pneumoniae, or as atypical, caused by Mycoplasma pneumoniae, Chlamydophila pneumoniae (formerly Chlamydia pneumoniae), Legionella species, and respiratory viruses. However, it is often not possible to distinguish typical versus atypical pneumonia solely on clinical grounds.
HISTORY AND PHYSICAL EXAMINATION
Common symptoms include fever (positive likelihood ratio [LR+] = 4.5), chills, pleuritic chest pain, and a cough producing mucopurulent sputum. Overall, physician judgment is moderately accurate for diagnosis of pneumonia, especially for ruling it out (LR+ = 2.0, negative likelihood ratio [LR–] = 0.24).7 Absence of fever and sputum also significantly reduces the likelihood of pneumonia in outpatients.8
High fever (greater than 104° F [40° C]), male sex, multilobar involvement, and gastrointestinal and neurologic abnormalities have been associated with CAP caused by Legionella infection.9 The clinical presentation of CAP is often more subtle in older patients, and many of these patients do not exhibit classic symptoms.1 They often present with weakness and decline in functional and mental status.
The patient history should focus on detecting symptoms consistent with CAP, underlying defects in host defenses, and possible exposure to specific pathogens. Persons with chronic obstructive pulmonary disease or human immunodeficiency virus infection have an increased incidence of CAP. Patients should be asked about occupation, animal exposures, and sexual history to help identify a specific infectious agent. A recent travel history (within two weeks) may help identify Legionella pneumonia, which has been associated with stays at hotels and on cruise ships. Influenza is often suggested on the basis of typical symptoms during peak influenza season.
Physical examination may reveal fever, dullness to percussion, egophony, tachycardia (LR+ = 2.1), and tachypnea (LR+ = 3.5). Asymmetric breath sounds, pleural rubs, egophony, and increased fremitus are relatively uncommon, but are highly specific for pneumonia (LR+ = 8.0); these signs help rule in pneumonia when present, but are not helpful when absent.8 Rales or bronchial breath sounds are helpful, but much less accurate than chest radiography.10 Tachypnea is common in older patients with CAP, occurring in up to 70 percent of those older than 65 years.11 Pulse oximetry screening should be performed in all patients with suspected CAP.12
RADIOLOGIC EXAMINATION
An infiltrate on lung imaging, usually chest radiography, is required for the diagnosis of CAP; therefore, the test should be performed in patients with clinically suspected CAP.12 Table 2 includes a tool for identifying patients with respiratory illness who would benefit from chest radiography.13 The extent of radiographic findings may help identify the severity of illness and assist with initial point-of-care decisions. Lobar consolidation, cavitation, and pleural effusions suggest a bacterial etiology. Diffuse parenchymal involvement is more often associated with Legionella or viral pneumonia. Because overuse of antibiotics for treatment of upper respiratory tract infections promotes drug resistance and can have adverse effects, identifying patients who will benefit from antimicrobial therapy is important.

| Chest radiography should be performed in: | |
| Any patient with at least one of the following abnormal vital signs: | |
| Temperature > 100° F (37.8° C) | |
| Heart rate > 100 beats per minute | |
| Respiratory rate > 20 breaths per minute | |
| Any patient with at least two of the following clinical findings: | |
| Decreased breath sounds | |
| Crackles (rales) | |
| Absence of asthma | |
LABORATORY TESTING
Routine laboratory testing to establish an etiology in outpatients with CAP is usually unnecessary. However, evaluation for specific pathogens that would alter standard empiric therapy should be performed when the presence of such pathogens is suspected on the basis of clinical and epidemiologic clues (Table 3).12 A randomized clinical trial comparing pathogen-driven therapy versus empiric therapy in patients with CAP found no statistically significant differences in mortality rate or length of hospitalization.14
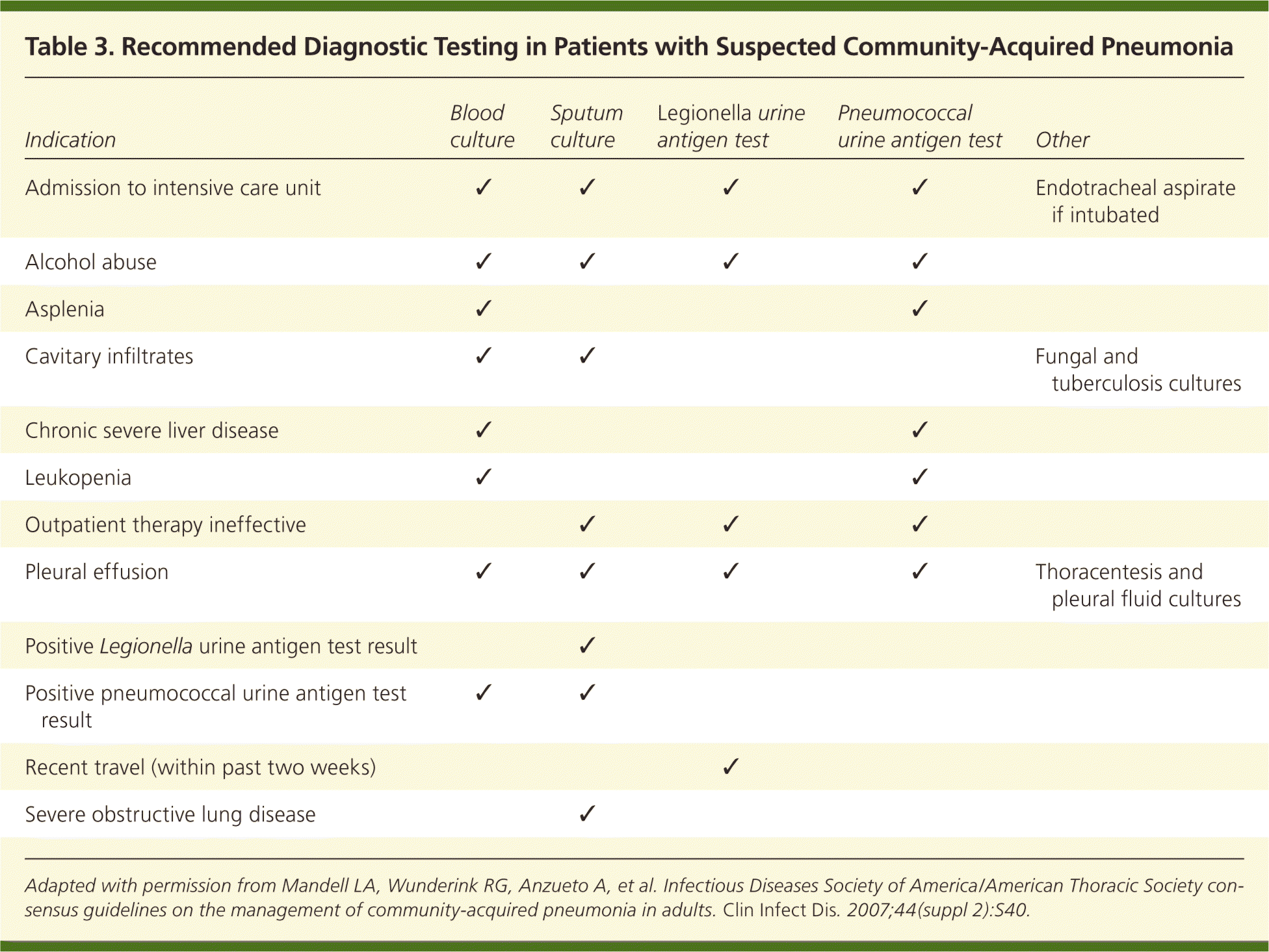
| Indication | Blood culture | Sputum culture | Legionella urine antigen test | Pneumococcal urine antigen test | Other |
|---|---|---|---|---|---|
| Admission to intensive care unit | ✓ | ✓ | ✓ | ✓ | Endotracheal aspirate if intubated |
| Alcohol abuse | ✓ | ✓ | ✓ | ✓ | |
| Asplenia | ✓ | ✓ | |||
| Cavitary infiltrates | ✓ | ✓ | Fungal and tuberculosis cultures | ||
| Chronic severe liver disease | ✓ | ✓ | |||
| Leukopenia | ✓ | ✓ | |||
| Outpatient therapy ineffective | ✓ | ✓ | ✓ | ||
| Pleural effusion | ✓ | ✓ | ✓ | ✓ | Thoracentesis and pleural fluid cultures |
| Positive Legionella urine antigen test result | ✓ | ||||
| Positive pneumococcal urine antigen test result | ✓ | ✓ | |||
| Recent travel (within past two weeks) | ✓ | ||||
| Severe obstructive lung disease | ✓ |
Hypoglycemia (blood glucose level less than 70 mg per dL [3.89 mmol per L]) at presentation is associated with increased 30-day mortality even after adjustment for other variables, including comorbid illness and Pneumonia Severity Index (PSI) score.15 Procalcitonin levels are elevated in many patients with bacterial infections, and several studies have shown procalcitonin tests to be potentially useful in CAP.16,17 However, the turnaround time for procalcitonin results can be prolonged, limiting their clinical usefulness. A white blood cell count greater than 10,400 per mm3 (10.40 × 109 per L; LR+ = 3.4, LR– = 0.52) and a C-reactive protein level of 5.0 mg per dL (47.62 nmol per L) or greater (LR+ = 3.1, LR– = 0.7) are modestly helpful when positive, but it is important to note that normal values do not rule out pneumonia.18
Blood cultures are not recommended for most hospitalized patients with CAP and should be performed according to the recommendations in Table 3.12 The most common blood isolate in patients with CAP is S. pneumoniae. A study comparing 125 patients with CAP caused by pneumococcal bacteremia and 1,847 patients with nonbacteremic CAP found no increase in poor outcomes among those with bacteremia.19 In addition, false-positive blood culture results have been associated with prolonged hospitalization and more vancomycin use.20 Blood cultures should be ordered for patients with severe CAP (Table 4) because they are more likely to be infected with bacteria other than S. pneumoniae.12 Blood cultures in patients with severe CAP have a higher yield, are more likely to grow pathogens not covered by empiric therapy, and have higher potential to influence antibiotic management.12

| Minor criteria |
| Blood urea nitrogen level = 20 mg per dL (7.14 mmol per L) |
| Confusion/disorientation |
| Hypotension requiring aggressive fluid resuscitation |
| Hypothermia (core temperature < 96.8° F [36° C]) |
| Leukopenia (white blood cell count < 4,000 per mm3 [4.00 × 109 per L]) |
| Multilobar infiltrates |
| PaO2/FiO2 ratio ≤ 250 |
| Respiratory rate ≥ 30 breaths per minute |
| Thrombocytopenia (platelet count < 100 × 103 per mm3 [100 × 109 per L]) |
| Major criteria |
| Invasive mechanical ventilation |
| Septic shock with need for vasopressors |
Infectious Diseases Society of America/American Thoracic Society (IDSA/ATS) guidelines recommend that sputum specimens be obtained before the initiation of antibiotic therapy in inpatients.12 A negative sputum culture result from a good-quality sample (i.e., positive for neutrophils, but less than 25 epithelial cells per low-power field) is strong evidence that gram-negative bacilli and Staphylococcus aureus are absent, and can prompt safe de-escalation of antibiotic therapy. Necrotizing or cavitary pneumonia may be caused by methicillin-resistant S. aureus (MRSA). Physicians should maintain a high clinical suspicion for MRSA pneumonia in patients with a history of MRSA skin lesions or other risk factors. In patients with suspected Legionella pneumonia, sputum culture can help identify a causative environmental exposure.12
Pleural effusions greater than 5 cm on lateral chest radiography should be drained by thoracentesis, and the fluid sent for Gram stain and aerobic and anaerobic cultures. Urine antigen tests are helpful when an adequate sputum culture is unobtainable or when antibiotic therapy has already been started. The sensitivity of the pneumococcal urine antigen test is 50 to 80 percent with a specificity of greater than 90 percent. Although the urine antigen test only detects Legionella serogroup 1, this serogroup causes 80 to 95 percent of CAP from Legionella; the test is 70 to 90 percent sensitive and 99 percent specific for serogroup 1. Urine antigen test results are positive on the first day of illness and remain positive for several weeks.12 In general, urine antigen tests are better at ruling in disease when positive; a negative test result does not rule out infection with a specific pathogen given its somewhat limited sensitivity.
Acute- and convalescent-phase serologic testing is the standard for other atypical causes of pneumonia. However, treating patients based on a positive acute-phase titer result has been shown to be unreliable.21 Therefore, serology for other atypical pathogens should not be routinely ordered. Rapid antigen testing or direct fluorescent antibody testing for influenza can help with consideration of antiviral therapy and may decrease use of antibacterial agents.12

| Prognostic variables* | ||
| Confusion | ||
| Blood urea nitrogen level > 20 mg per dL (7.14 mmol per L) | ||
| Respiratory rate ≥ 30 breaths per minute | ||
| Blood pressure (systolic < 90 mm Hg or diastolic ≤ 60 mm Hg) | ||
| Age ≥ 65 years | ||
| Score | Inpatient vs. outpatient | 30-day mortality (%) |
| 0 or 1 point | Treat as outpatient | 0.7 to 2.1 |
| 2 points | Treat as inpatient | 9.2 |
| ≥3 points | Treat in intensive care unit | 15 to 40 |
Management
The initial management of CAP depends on the patient's severity of illness; underlying medical conditions and risk factors, such as smoking; and ability to adhere to a treatment plan. The need for hospitalization is the first decision that needs to be made after CAP is diagnosed or suspected.
INPATIENT VS. OUTPATIENT CARE
The estimated direct cost of a single CAP hospitalization ranges from $3,000 to $13,000.6 Patients admitted to the hospital are at risk of hospital-acquired complications, such as thromboembolic events, superinfections (e.g., Clostridium difficile colitis), and catheter-associated urinary tract infections. Mortality and severity prediction scores have been designed to identify patients with CAP who can be treated safely as outpatients. The PSI is the most extensively validated prediction score, but it is limited by its complexity and failure to always recognize the most severely ill patients, especially those without comorbid illness.22
Table 5 summarizes the CURB-65 (confusion, uremia, respiratory rate, blood pressure), a prediction score developed by the British Thoracic Society.1 It is simpler than the PSI but does not specifically account for decompensated chronic illness that occurs with CAP. CURB-65 has been shown to predict death from CAP in hospital and outpatient settings.23
More recently, SMART-COP (systolic blood pressure, multilobar chest radiography, albumin level, respiratory rate, tachycardia, confusion, oxygen level, arterial pH) was created to predict which patients will require intensive respiratory or vasopressor support (Table 6).24 A SMART-COP score of 3 or more points identifies 92 percent of those who will receive intensive respiratory or vasopressor support, whereas sensitivities for PSI and CURB-65 are 74 and 39 percent, respectively.24 Patients admitted to the intensive care unit (ICU) with CAP are more likely to be men who have congestive heart failure or chronic obstructive pulmonary disease.25

| Variable | Points | |
|---|---|---|
| Systolic blood pressure < 90 mm Hg | 2 | |
| Multilobar involvement on chest radiography | 1 | |
| Albumin level < 3.5 g per dL (35 g per L) | 1 | |
| Respiratory rate | 1 | |
| 50 years and younger: ≥ 25 breaths per minute | ||
| Older than 50 years: ≥ 30 breaths per minute | ||
| Tachycardia (≥ 125 beats per minute) | 1 | |
| Confusion (new onset) | 1 | |
| Oxygen level | 2 | |
| 50 years and younger: PaO2 < 70 mm Hg, oxygen saturation ≤ 93 percent, or (if on oxygen) PaO2/FiO2 ratio < 333 | ||
| Older than 50 years: PaO2 < 60 mm Hg, oxygen saturation ≤90 percent, or (if on oxygen) PaO2/FiO2 ratio < 250 | ||
| Arterial pH < 7.35 | 2 | |
| Total:_____ | ||
ANTIBIOTIC THERAPY
Because the exact causative organism is not identified in many patients with CAP, treatment is usually empiric. Recommendations for antibiotic therapy in these patients are listed in Table 7.12 One of the major differences between U.S. and European guidelines for treatment of CAP is that all patients in the United States receive treatment for S. pneumoniae and atypical organisms because CAP is more often caused by these pathogens in North America.26 Macrolides (e.g., azithromycin [Zithromax], clarithromycin [Biaxin]) can be used for outpatients with no cardiopulmonary disease or recent antibiotic use.

| Patient group | Initial therapy | ||
|---|---|---|---|
| Previously healthy outpatients; no antibiotic use in past three months | A macrolide or doxycycline | ||
| Outpatients with comorbidities* or antibiotic use in past three months† | A respiratory fluoroquinolone (levofloxacin [Levaquin], gemifloxacin [Factive], or moxifloxacin [Avelox]), or a beta-lactam antibiotic (high-dose amoxicillin, amoxicillin/clavulanate [Augmentin], or cefpodoxime) plus a macrolide‡ | ||
| Inpatients, non-ICU | A respiratory fluoroquinolone, or a beta-lactam antibiotic plus a macrolide | ||
| Inpatients, ICU | A beta-lactam antibiotic (ceftriaxone [Rocephin], cefotaxime [Claforan], or ampicillin/sulbactam [Unasyn]), plus azithromycin (Zithromax) or a respiratory fluoroquinolone§ | ||
| Special considerations | |||
| Risk factors for Pseudomonas species | A beta-lactam antibiotic (piperacillin/tazobactam [Zosyn], cefepime, imipenem/cilastatin [Primaxin], meropenem [Merrem], or doripenem [Doribax]), plus either ciprofloxacin (Cipro) or levofloxacin | ||
| or | |||
| The above beta-lactam antibiotic plus an aminoglycoside and azithromycin | |||
| or | |||
| The above beta-lactam antibiotic plus an aminoglycoside and an antipneumococcal respiratory fluoroquinolone | |||
| Risk factors for methicillin-resistant Staphylococcus aureus | Vancomycin or linezolid (Zyvox) | ||
| Influenza virus | Oseltamivir (Tamiflu) or zanamivir (Relenza) | ||
Drug-resistant S. pneumoniae is a concern in patients with comorbid illness or recent antibiotic therapy (within previous three months) and should be treated with an oral beta-lactam antibiotic (e.g., high-dose amoxicillin, amoxicillin/clavulanate [Augmentin], cefpodoxime) combined with a macrolide. A respiratory fluoroquinolone is another choice. If a patient has used an antibiotic in the previous three months, a drug from a different class should be prescribed to decrease the risk of pneumococcal resistance. For hospitalized patients not admitted to the ICU, an intravenous respiratory fluoroquinolone alone or an intravenous beta-lactam antibiotic combined with a macrolide or doxycycline should be given. A study showed doxycycline to be comparable to levofloxacin (Levaquin) in effectiveness, length of hospital stay, and failure rate for empiric treatment of CAP; doxycycline is also a less expensive option for hospitalized patients who are not admitted to the ICU.27 However, the sample size in the study was small and IDSA/ATS guidelines recommend doxycycline only for outpatients.12
All patients with CAP who are admitted to the ICU should be treated with dual therapy, which is associated with lower mortality from bacteremic pneumococcal pneumonia and improves survival in patients with CAP and shock.28 Some patients with severe CAP, especially after an episode of influenza or viral illness, who are admitted to the ICU need added coverage for S. aureus, including MRSA. MRSA-associated CAP is characterized by a severe, bilateral, necrotizing pneumonia induced by Panton-Valentine leukocidin and other toxins.
Duration of therapy for patients with CAP has traditionally been 10 to 14 days, but more recent evidence suggests a shorter course of up to seven days is equally effective.29 Hospitalized patients may be switched from intravenous to oral antibiotic therapy after they have clinical improvement and are able to tolerate oral medications. An early switch from intravenous to oral antibiotics after three days in patients with severe CAP has been shown to be effective and may decrease length of hospital stay.30 A course of oral azithromycin after completing intravenous azithromycin and ceftriaxone (Rocephin) is effective and well-tolerated.31 Treatment of patients who do not respond well to initial treatment is summarized in Table 8.12

| Scenario | Considerations* |
|---|---|
| Delayed response to therapy with no improvement after 72 hours | Resistant microorganism or uncovered pathogen |
| Parapneumonic effusion or empyema | |
| Nosocomial superinfection | |
| Noninfectious condition, such as pulmonary embolism, drug fever, bronchiolitis obliterans, organizing pneumonia, congestive heart failure, vasculitis | |
| Clinical deterioration or continued progression of illness | Severity of illness at presentation |
| Metastatic infection, such as empyema, endocarditis, meningitis, arthritis | |
| Inaccurate diagnosis, such as acute respiratory distress syndrome, aspiration | |
| Exacerbation of comorbid illness or coexisting noninfectious disease, such as renal failure, acute myocardial infarction, pulmonary embolism |
ADJUNCTIVE THERAPIES
Quality Improvement and Prevention
The Centers for Medicare and Medicaid Services has developed a set of core measures for CAP that is collected for every hospital and reported on the Hospital Compare Web site (http://www.healthcare.gov/compare). Adhering to national guidelines has been shown to improve length of hospital stay and other outcomes33,34; however, they do not take into account individual patient differences and should not supplant physician judgment. Pneumococcal vaccination is recommended for all persons 65 years and older, adults younger than 65 years who have chronic illness or asplenia, and all adults who smoke or have asthma.35 However, effectiveness may decrease with age, and studies evaluating its effectiveness against pneumonia without bacteremia have been mixed.36–38 The influenza vaccine is also important for the prevention of CAP. However, its effectiveness is influenced by host factors and how closely the antigens in the vaccine are matched with the circulating influenza strain.12 The influenza vaccine has also been shown to effectively prevent pneumonia, hospitalization, and death in older persons.39
Data Sources: A PubMed search was completed in Clinical Queries using the key term community-acquired pneumonia. The search included meta-analyses, randomized controlled trials, clinical trials, practice guidelines, and reviews. The limits included English language, humans, and all adults 19 years and older. We also searched the National Guideline Clearinghouse, Agency for Healthcare Research and Quality Evidence Reports, Cochrane Database of Systematic Reviews, and the U.S. Preventive Services Task Force. Search date: September 19, 2010.
