Am Fam Physician. 2011;84(6):653-660
Author disclosure: Dr. Scott Horowitz and his wife, Dr. Diane Horowitz, disclose that he is on the speakers' bureaus of Merck/Schering-Plough (Avelox) and Pfizer (Vfend and Zyvox). However, none of the drugs that are the basis of his affiliations are mentioned in this article, and the topic is not directly related to his affiliations. The other authors have no relevant financial affiliations to disclose.
Prompt diagnosis and treatment of infectious arthritis can help prevent significant morbidity and mortality. The acute onset of monoarticular joint pain, erythema, heat, and immobility should raise suspicion of sepsis. Constitutional symptoms such as fever, chills, and rigors are poorly sensitive for septic arthritis. In the absence of peripheral leukopenia or prosthetic joint replacement, synovial fluid white blood cell count in patients with septic arthritis is usually greater than 50,000 per mm3. Isolation of the causative agent through synovial fluid culture is not only definitive but also essential before selecting antibiotic therapy. Synovial fluid analysis is also useful to help distinguish crystal arthropathy from infectious arthritis, although the two occasionally coexist. Almost any microorganism can be pathogenic in septic arthritis; however, septic arthritis is caused by nongonococcal pathogens (most commonly Staphylococcus species) in more than 80 percent of patients. Gram stain results should guide initial antibiotic choice. Vancomycin can be used for gram-positive cocci, ceftriaxone for gram-negative cocci, and ceftazidime for gram-negative rods. If the Gram stain is negative, but there is strong clinical suspicion for bacterial arthritis, treatment with vancomycin plus ceftazidime or an aminoglycoside is appropriate. Evacuation of purulent material with arthrocentesis or surgical methods is necessary. Special consideration should be given to patients with prosthetic joint infection. In this population, the intraarticular cutoff values for infection may be as low as 1,100 white blood cells per mm3 with a neutrophil differential of greater than 64 percent.
Septic arthritis is a key consideration in adults presenting with acute monoarticular arthritis. Failure to initiate appropriate antibiotic therapy within the first 24 to 48 hours of onset can cause subchondral bone loss and permanent joint dysfunction.1,2 The incidence of septic arthritis ranges widely, between four and 29 cases per 100,000 person-years, and depends on population variables and preexisting structural joint abnormalities.1
Because of the lack of a limiting basement plate in synovial tissues, the most common route of entry into the joint is hematogenous spread during bacteremia.3–7 Pathogens may also enter through direct inoculation (e.g., arthrocentesis, arthroscopy, trauma) or contiguous spread from local infections (e.g., osteomyelitis, septic bursitis, abscess).3–5 Once in the joint, microorganisms are deposited in the synovial membrane, causing an acute inflammatory response.2,7 Inflammatory mediators and pressure from large effusions lead to the destruction of joint cartilage and bone loss.2,7 A history, physical examination, and joint fluid analysis are warranted to ensure timely joint-preserving interventions.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Suspicion of septic arthritis should be pursued with arthrocentesis, and synovial fluid should be sent for white blood cell count, crystal analysis, Gram stain, and culture. | C | 21–23 |
| In addition to antibiotic therapy, evacuation of purulent material is necessary in patients with septic arthritis; arthrocentesis and surgical methods are appropriate. | C | 48 |
| Intraarticular white blood cell cutoff values for infection as low as 1,100 per mm3 (1.10 × 109 per L) with a neutrophil differential of greater than 64 percent can help diagnose prosthetic joint infection. | C | 66 |
Diagnosis
HISTORY
Patients presenting with acute joint swelling, pain, erythema, warmth, and joint immobility should be screened for risk factors associated with septic arthritis (Table 18–12 ). A prospective study in the Netherlands of patients diagnosed with septic arthritis found that 84 percent of adults had an underlying medical condition and 59 percent had a previous joint disorder.13 In a review of musculoskeletal infections in patients with human immunodeficiency virus infection, about 0.5 percent of hospitalized patients had septic arthritis, regardless of CD4 lymphocyte count or disease stage.14 Particular vigilance is needed during a monoarticular flare-up of rheumatoid arthritis, because patients on immunosuppressive medications, but not biologic therapy, have a fourfold increased risk of septic arthritis.8 Many patients with rheumatoid arthritis are treated with anti–tumor-necrosis-factor-α, which further increases the risk of infection twofold.15
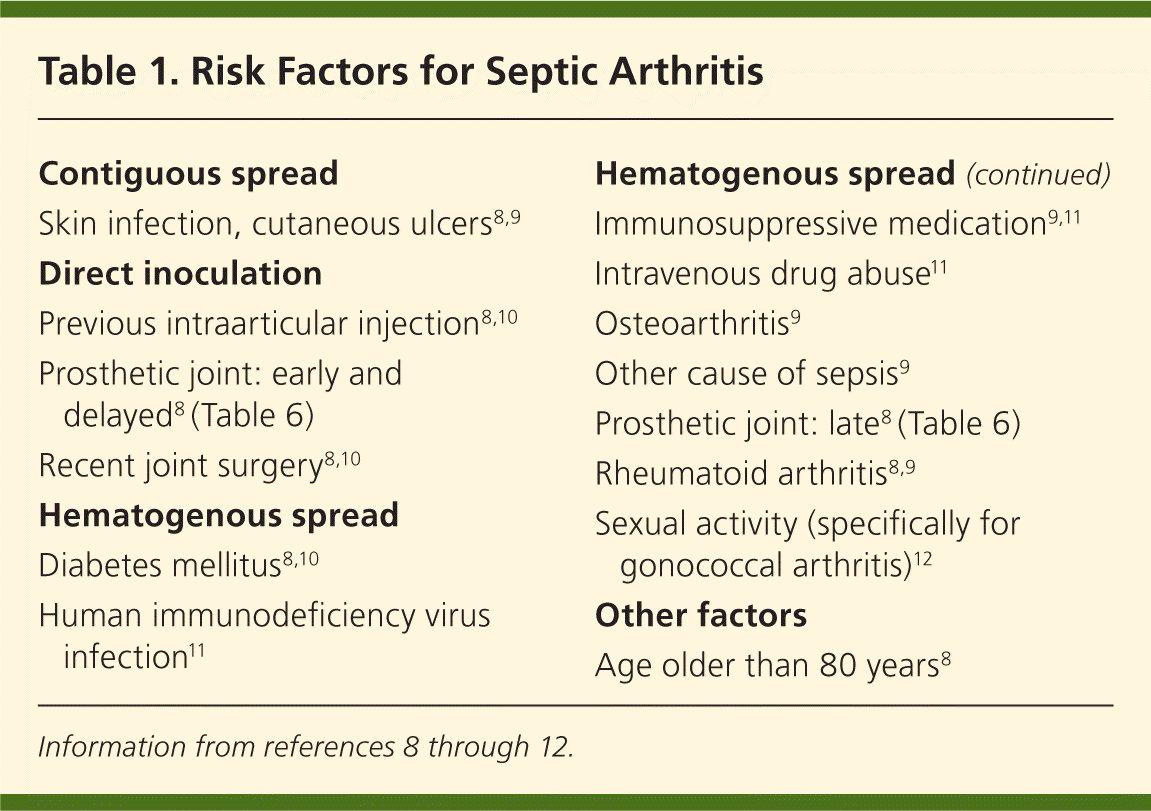
| Contiguous spread |
| Skin infection, cutaneous ulcers8,9 |
| Direct inoculation |
| Previous intraarticular injection8,10 |
| Prosthetic joint: early and delayed8 (Table 6) |
| Recent joint surgery8,10 |
| Hematogenous spread |
| Diabetes mellitus8,10 |
| Human immunodeficiency virus infection11 |
| Hematogenous spread |
| Immunosuppressive medication9,11 |
| Intravenous drug abuse11 |
| Osteoarthritis9 |
| Other cause of sepsis9 |
| Prosthetic joint: late8 (Table 6) |
| Rheumatoid arthritis8,9 |
| Sexual activity (specifically for gonococcal arthritis)12 |
| Other factors |
| Age older than 80 years8 |
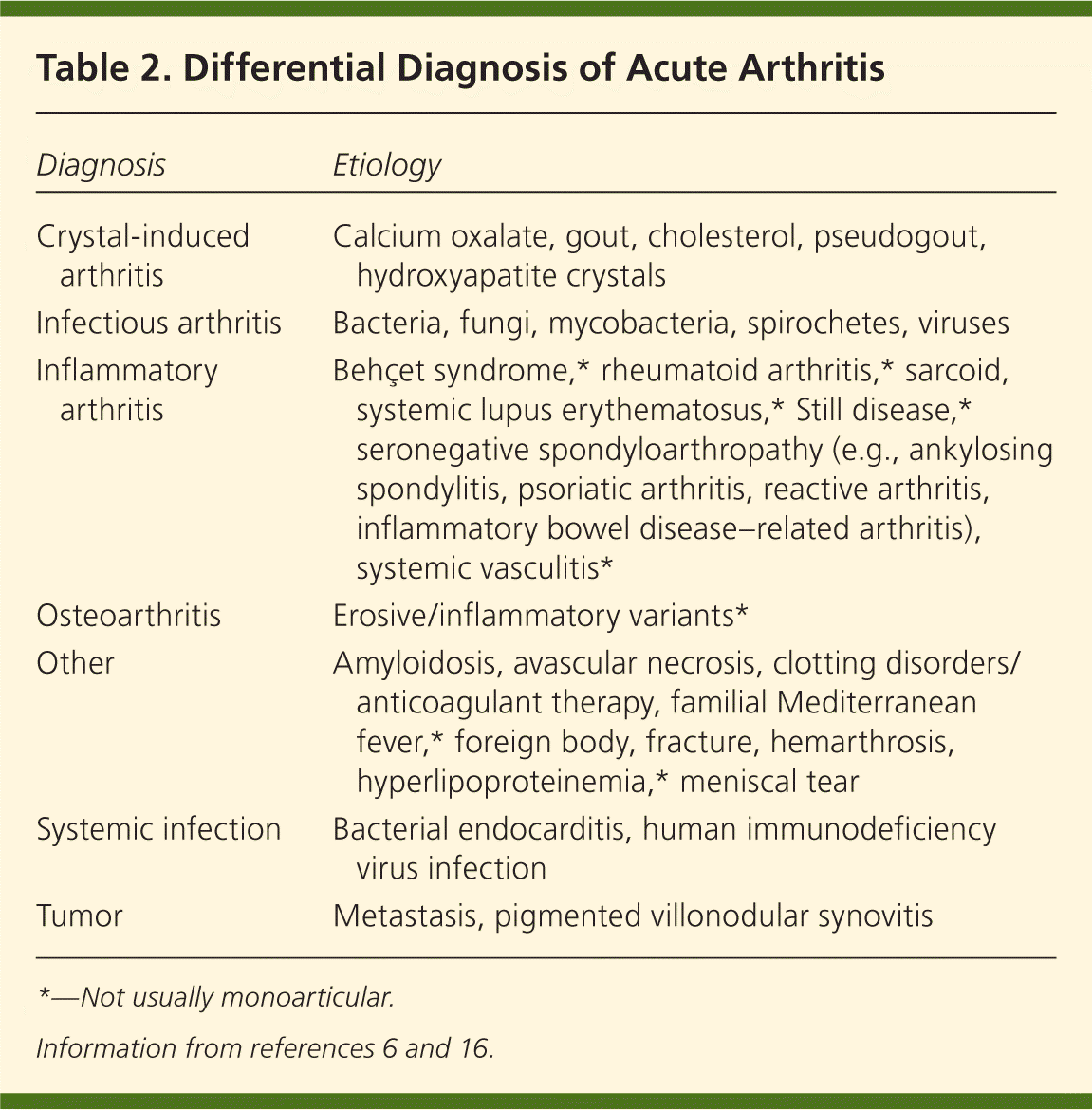
| Diagnosis | Etiology |
|---|---|
| Crystal-induced arthritis | Calcium oxalate, gout, cholesterol, pseudogout, hydroxyapatite crystals |
| Infectious arthritis | Bacteria, fungi, mycobacteria, spirochetes, viruses |
| Inflammatory arthritis | Behçet syndrome,* rheumatoid arthritis,* sarcoid, systemic lupus erythematosus,* Still disease,* seronegative spondyloarthropathy (e.g., ankylosing spondylitis, psoriatic arthritis, reactive arthritis, inflammatory bowel disease–related arthritis), systemic vasculitis* |
| Osteoarthritis | Erosive/inflammatory variants* |
| Other | Amyloidosis, avascular necrosis, clotting disorders/anticoagulant therapy, familial Mediterranean fever,* foreign body, fracture, hemarthrosis, hyperlipoproteinemia,* meniscal tear |
| Systemic infection | Bacterial endocarditis, human immunodeficiency virus infection |
| Tumor | Metastasis, pigmented villonodular synovitis |
PHYSICAL EXAMINATION
The physical examination should determine if the site of inflammation is intraarticular or periarticular, such as a bursa or skin. Generally, intraarticular pathology results in severe limitation of active and passive range of motion, and the joint is often held in the position of maximal intraarticular space. For example, a septic knee will be extended fully. Conversely, pain from periarticular pathology occurs only during active range of motion, and swelling will be more localized.
Although septic arthritis is usually monoarticular, up to 20 percent of cases are oligoarticular.1 In native joints, the knee is the most commonly affected, followed by the hip, shoulder, ankle, elbow, and wrist.3,4,17,18 Infections of axial joints, such as the sternoclavicular or sacroiliac joint, may occur; however, they are more common in patients with a history of intravenous drug abuse.11,19
LABORATORY EVALUATION
Serum markers, such as white blood cell (WBC) count, erythrocyte sedimentation rate,10,17 and C-reactive protein levels,9 are often used to determine the presence of infection or inflammatory response. Patients with confirmed septic arthritis have been found to have normal erythrocyte sedimentation rates and C-reactive protein levels.9 When elevated, these markers may be used to monitor therapeutic response. Because pathogenesis may be hematogenous, blood cultures are positive in 25 to 50 percent of patients with septic arthritis.1,9,20
SYNOVIAL FLUID ANALYSIS
Because the clinical presentation of septic arthritis may overlap with other causes of acute arthritis (Table 26,16 ), arthrocentesis is needed to identify the causative infectious agent. Synovial fluid should be evaluated at the bedside and then sent for WBC count with differential, crystal analysis, Gram stain, and culture21–23 (Table 319–21,24–26 ). In synovial fluid, a WBC count of more than 50,000 per mm3 (50 × 109 per L) and a polymorphonuclear cell count greater than 90 percent have been directly correlated with infectious arthritis, although this overlaps with crystalline disease.1,6 Lower synovial fluid WBC counts may occur in persons with disseminated gonococcal disease, peripheral leukopenia, or joint replacement.6,19,22 Septic arthritis can coexist with crystal arthropathy; therefore, the presence of crystals does not preclude a diagnosis of septic arthritis.27
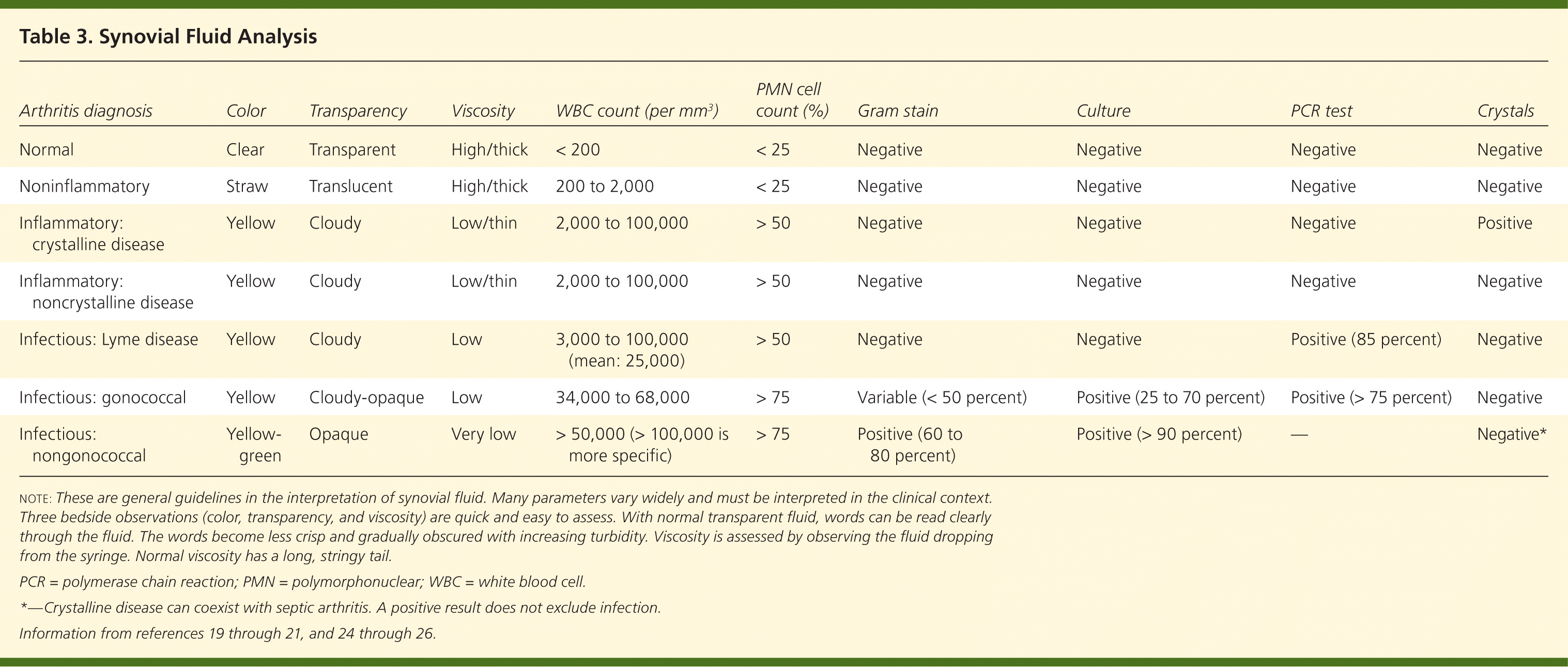
| Arthritis diagnosis | Color | Transparency | Viscosity | WBC count (per mm3 ) | PMN cell count (%) | Gram stain | Culture | PCR test | Crystals |
|---|---|---|---|---|---|---|---|---|---|
| Normal | Clear | Transparent | High/thick | < 200 | < 25 | Negative | Negative | Negative | Negative |
| Noninflammatory | Straw | Translucent | High/thick | 200 to 2,000 | < 25 | Negative | Negative | Negative | Negative |
| Inflammatory: crystalline disease | Yellow | Cloudy | Low/thin | 2,000 to 100,000 | > 50 | Negative | Negative | Negative | Positive |
| Inflammatory: noncrystalline disease | Yellow | Cloudy | Low/thin | 2,000 to 100,000 | > 50 | Negative | Negative | Negative | Negative |
| Infectious: Lyme disease | Yellow | Cloudy | Low | 3,000 to 100,000 (mean: 25,000) | > 50 | Negative | Negative | Positive (85 percent) | Negative |
| Infectious: gonococcal | Yellow | Cloudy-opaque | Low | 34,000 to 68,000 | > 75 | Variable (< 50 percent) | Positive (25 to 70 percent) | Positive (> 75 percent) | Negative |
| Infectious: nongonococcal | Yellow-green | Opaque | Very low | > 50,000 (> 100,000 is more specific) | > 75 | Positive (60 to 80 percent) | Positive (> 90 percent) | — | Negative* |
Measuring synovial fluid glucose or protein is not useful because results are neither sensitive nor specific for septic arthritis.6 Polymerase chain reaction (PCR) testing may help isolate less common organisms, such as Borrelia species,24,25 and should be ordered if there is a high level of clinical suspicion. The sensitivities of synovial fluid, Gram stain, and culture vary by pathogenic organism.
IMAGING
There are no data on imaging studies that are pathognomonic for acute septic arthritis. Plain films establish a baseline and may detect fractures, chondrocalcinosis, or inflammatory arthritis. Ultrasonography is more sensitive for detecting effusions, particularly in difficult-to-examine joints, such as the hip.28 Magnetic resonance imaging findings that suggest an acute intraarticular infection include the combination of bone erosions with marrow edema.29 Imaging may allow guided arthrocentesis, particularly in difficult-to-examine joints (e.g., hip, sacroiliac, costochondral).
Organisms
Almost any microorganism may be pathogenic in septic arthritis. Bacterial causes of septic arthritis include staphylococci (40 percent), streptococci (28 percent), gram-negative bacilli (19 percent), mycobacteria (8 percent), gram-negative cocci (3 percent), gram-positive bacilli (1 percent), and anaerobes (1 percent).30 There are various characteristic presentations depending on the pathogen, underlying medical conditions, or exposures (Table 47,18,20,31–34 ). Clinical presentations can be broadly grouped into three categories: nongonococcal, gonococcal, and other (e.g., Lyme disease, mycobacterial, fungal).
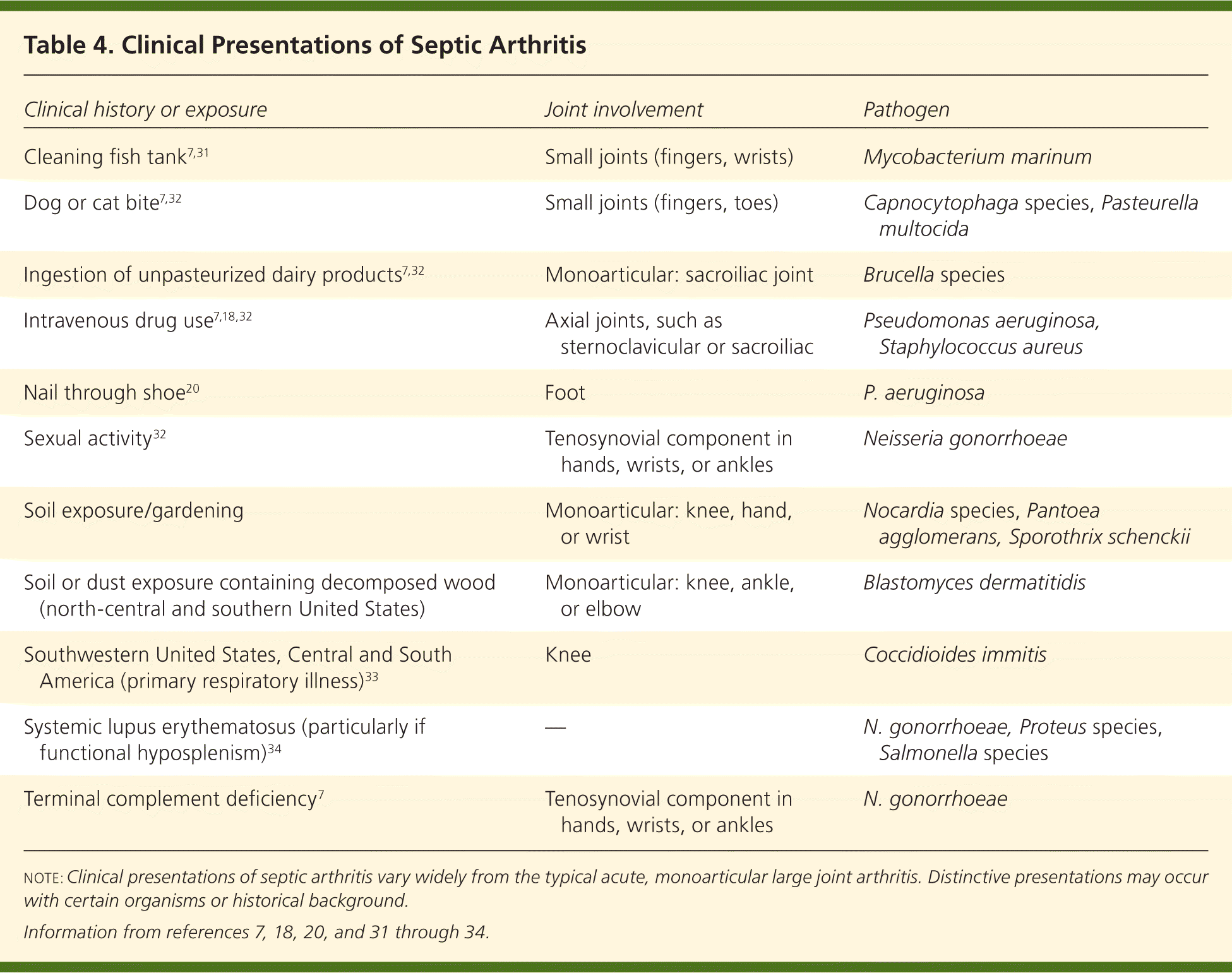
| Clinical history or exposure | Joint involvement | Pathogen |
|---|---|---|
| Cleaning fish tank7,31 | Small joints (fingers, wrists) | Mycobacterium marinum |
| Dog or cat bite7,32 | Small joints (fingers, toes) | Capnocytophaga species, Pasteurella multocida |
| Ingestion of unpasteurized dairy products7,32 | Monoarticular: sacroiliac joint | Brucella species |
| Intravenous drug use7,18,32 | Axial joints, such as sternoclavicular or sacroiliac | Pseudomonas aeruginosa, Staphylococcus aureus |
| Nail through shoe20 | Foot | P. aeruginosa |
| Sexual activity32 | Tenosynovial component in hands, wrists, or ankles | Neisseria gonorrhoeae |
| Soil exposure/gardening | Monoarticular: knee, hand, or wrist | Nocardia species, Pantoea agglomerans, Sporothrix schenckii |
| Soil or dust exposure containing decomposed wood (north-central and southern United States) | Monoarticular: knee, ankle, or elbow | Blastomyces dermatitidis |
| Southwestern United States, Central and South America (primary respiratory illness)33 | Knee | Coccidioides immitis |
| Systemic lupus erythematosus (particularly if functional hyposplenism)34 | — | N. gonorrhoeae, Proteus species, Salmonella species |
| Terminal complement deficiency7 | Tenosynovial component in hands, wrists, or ankles | N. gonorrhoeae |
NONGONOCOCCAL ARTHRITIS
Septic arthritis is caused by nongonococcal pathogens in more than 80 percent of patients.2 Nongonococcal arthritis often affects older persons, is acute in nature, and is monoarticular in more than 80 percent of patients.12 Synovial fluid cultures are positive in more than 90 percent of patients with nongonococcal arthritis, as opposed to blood cultures, which are positive in only 50 percent of patients.12,30
Gram-positive staphylococci and streptococci are the causative agents in the majority of bacterial arthritis cases in which an organism is identified,3 and are associated with drug abuse, cellulitis, abscesses, endocarditis, and chronic osteomyelitis.18 Staphylococcus aureus is the organism most commonly found in patients with septic arthritis in the United States and other developed countries,30,35 and Streptococcus species is the next most common.30,36 The presence of methicillin-resistant S. aureus (MRSA) is an emerging clinical problem. Although data are mostly limited to case reports, the incidence of MRSA ranges between 5 and 25 percent of bacterial arthritis cases, and tends to affect older persons, involve the shoulder, and the health care–associated MRSA strain.37,38
Gram-negative bacilli represent approximately 14 to 19 percent of septic arthritis cases12,18 and are associated with invasive urinary tract infections, intravenous drug use, older age, compromised immune system, and skin infections.18 The two most common gram-negative organisms detected in adults are Pseudomonas aeruginosa and Escherichia coli.10,12,17,18 Historically, Haemophilus influenzae infection has occurred more often in children,2,39 although this may be tempered by widespread H. influenzae type b vaccination.40
GONOCOCCAL ARTHRITIS
Patients with disseminated Neisseria gonorrhoeae infection are usually young, healthy, and sexually active.30 Disseminated gonococcal infection may have various clinical musculoskeletal presentations, with or without associated dermatitis. Patients typically display a migratory pattern of arthralgias, tenosynovial inflammation, or nonerosive arthritis.6,23,30 Blood cultures are seldom positive, and synovial fluid cultures are variable, with a positive result in only 25 to 70 percent of patients with gonococcal arthritis.19,23 When a disseminated gonococcal infection is suspected, cultures should be taken from potentially infected mucosal sites (e.g., urethra, rectum, pharynx, cervix).30,41 PCR testing has a sensitivity of 76 percent and a specificity of 96 percent for N. gonorrhoeae, and may be useful in patients with culture-negative disease if the clinical scenario is unclear or similar to a reactive arthritis.26
OTHER INFECTIONS
Mycobacterial infectious arthritis is also indolent, which can cause a considerable delay in diagnosis, although joint damage does not occur as rapidly as it does in bacterial infections.31 Articular Mycobacterium tuberculosis infection typically affects the hip or knee; is usually caused by reactivation from past dissemination; and may occur without other manifestations of active tuberculosis.31,42 Synovial fluid culture is positive in 80 percent of patients with M. tuberculosis infection.34 Acid-fast smears are not helpful and are often negative.31 Histology is not specific because it may mimic other granulomatous diseases, although synovial biopsy will be positive for M. tuberculosis in about 95 percent of cases.34
Borrelia burgdorferi infection initially causes viral-like migratory arthralgias of Lyme disease. Late disease is characterized by an intermittent oligoarthritis that usually involves the knee or other large joints.44 The diagnosis of Lyme arthritis can be made with a two-step serologic testing process involving enzyme-linked immunosorbent assay, followed by confirmation with a Western blot or immunoblot test.45 B. burgdorferi cannot be cultured from synovial fluid46; however, PCR testing is positive in 85 percent of patients with Lyme arthritis, making it a confirmatory test.47 It should be noted that PCR testing cannot distinguish live from dead organisms.46
Management
Empiric intravenous antibiotic treatment of septic arthritis should be based on the organism found in the Gram stain of the synovial fluid, or on the suspicion of a pathogen from the patient's clinical presentation (Table 47,18,20,31–34 ). Treatment options include vancomycin for gram-positive cocci, ceftriaxone (Rocephin) for gram-negative cocci, and ceftazidime (Fortaz) for gram-negative rods (Table 532 ). If the Gram stain is negative but there is suspicion of bacterial arthritis, vancomycin plus either ceftazidime or an aminoglycoside is appropriate.32 Adjustments to the administration route and the duration of treatment should be based on the clinical response and microbiology results. The Sanford Guide (http://www.sanfordguide.com; subscription required) is a resource for the management of infectious syndromes. It includes dosing options for patients with renal insufficiency and drug allergies, and lists common drug interactions.
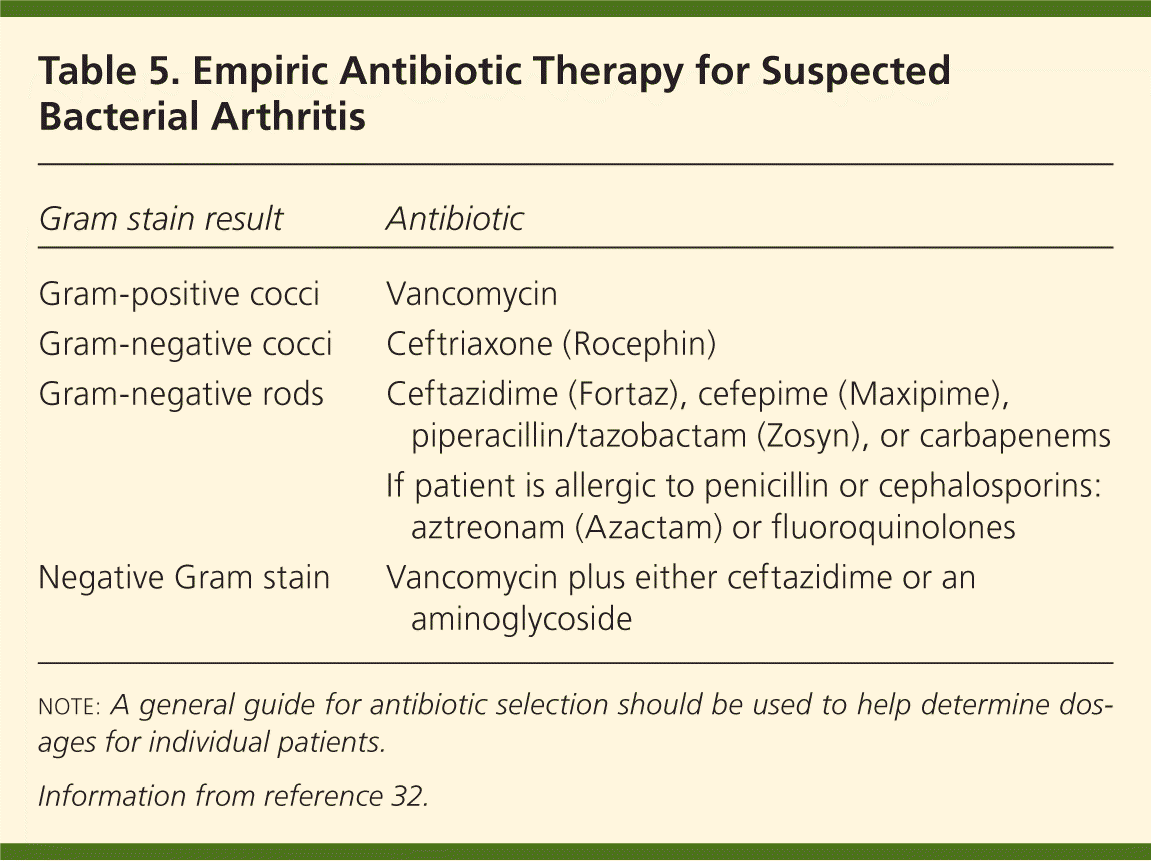
| Gram stain result | Antibiotic |
|---|---|
| Gram-positive cocci | Vancomycin |
| Gram-negative cocci | Ceftriaxone (Rocephin) |
| Gram-negative rods | Ceftazidime (Fortaz), cefepime (Maxipime), piperacillin/tazobactam (Zosyn), or carbapenems |
| If patient is allergic to penicillin or cephalosporins: aztreonam (Azactam) or fluoroquinolones | |
| Negative Gram stain | Vancomycin plus either ceftazidime or an aminoglycoside |
The duration of therapy in patients with nongonococcal septic arthritis is typically three to four weeks. Therapy for disseminated gonococcal infection involves a third-generation cephalosporin, such as ceftriaxone, for 24 to 48 hours after improvement begins, followed by oral therapy.41 The clinical response should be rapid, with symptoms improving within 24 to 48 hours. Treatment then may be switched to oral cefixime (Suprax), or ciprofloxacin (Cipro) if quinolone resistance is not a concern, for at least one week.41 Treatment of chlamydia also should be considered in the absence of appropriate testing.41 Treatment of fungal arthritis is species-dependent, but usually includes an oral azole or parenteral amphotericin B.42,43 Lyme arthritis responds well to parenteral ceftriaxone or oral doxycycline.47
In addition to antibiotic therapy, evacuation of purulent material is necessary. Guidelines from 2006 do not distinguish between arthrocentesis and surgical methods.48 Repeated daily joint aspirations are successful during the first five days of treatment.49 With each aspirate, synovial fluid WBC count, polymorphonuclear cell count, Gram stain, and culture should be evaluated to ensure clinical response. Open or arthroscopic techniques can be used to surgically drain the infected joint. Arthroscopic drainage is associated with rapid recovery and low morbidity.50 Direct visualization of the joint tissue facilitates the lysis of adhesions, drainage of purulent pockets, and debridement of necrotic material.
Prognosis
Before antibiotics were available, two-thirds of patients died from septic arthritis.51 Current mortality rates of bacterial arthritis range from 10 to 20 percent, depending on the presence of comorbid conditions, such as older age, coexisting renal or cardiac disease, and concurrent immunosuppression.3,9,52 Factors associated with death include age 65 years or older, and infection in the shoulder, elbow, or at multiple sites.52
After completing antimicrobial therapy, patients with S. aureus septic arthritis regain 46 to 50 percent of their baseline joint function.9 In contrast, adults with pneumococcal septic arthritis who survive infection (the mortality rate is approximately 20 percent) will return to 95 percent of their baseline joint function after completing antimicrobial therapy.3 Morbidity (e.g., amputation, arthrodesis, prosthetic surgery, severe functional deterioration) occurs in one-third of patients with bacterial arthritis, usually affecting older patients, those with preexisting joint disease, and those with synthetic intraarticular material.52
Special Considerations
Prosthetic joint infections occur in 0.86 to 1.1 percent of knee arthroplasties53,54 and in 0.3 to 1.7 percent of hip arthroplasties.53,55 These infections may result in failure of the joint replacement. In prosthetic joint infections, bacterial adherence to prosthetic surfaces forms biofilms, which can lead to increased resistance to the host's immune system and to antimicrobials.56–58
Prosthetic joint infections are usually caused by gram-positive cocci, including coagulase-negative staphylococci and S. aureus.53,54 Other organisms, such as gram-negative bacilli53,59 and mycobacteria,60 have also been implicated. Polymicrobial infections after hip and knee arthroplasties have been reported in up to 20 percent of patients.61 Risk factors for the development of prosthetic joint infections include previous fracture, seropositive rheumatoid arthritis, high body mass index, revision arthroplasty, and surgical site infections.59,61,62 Table 619,63–65 lists the three categories of prosthetic joint infections with typical pathogenesis, timing, clinical presentation, and mode of infection.
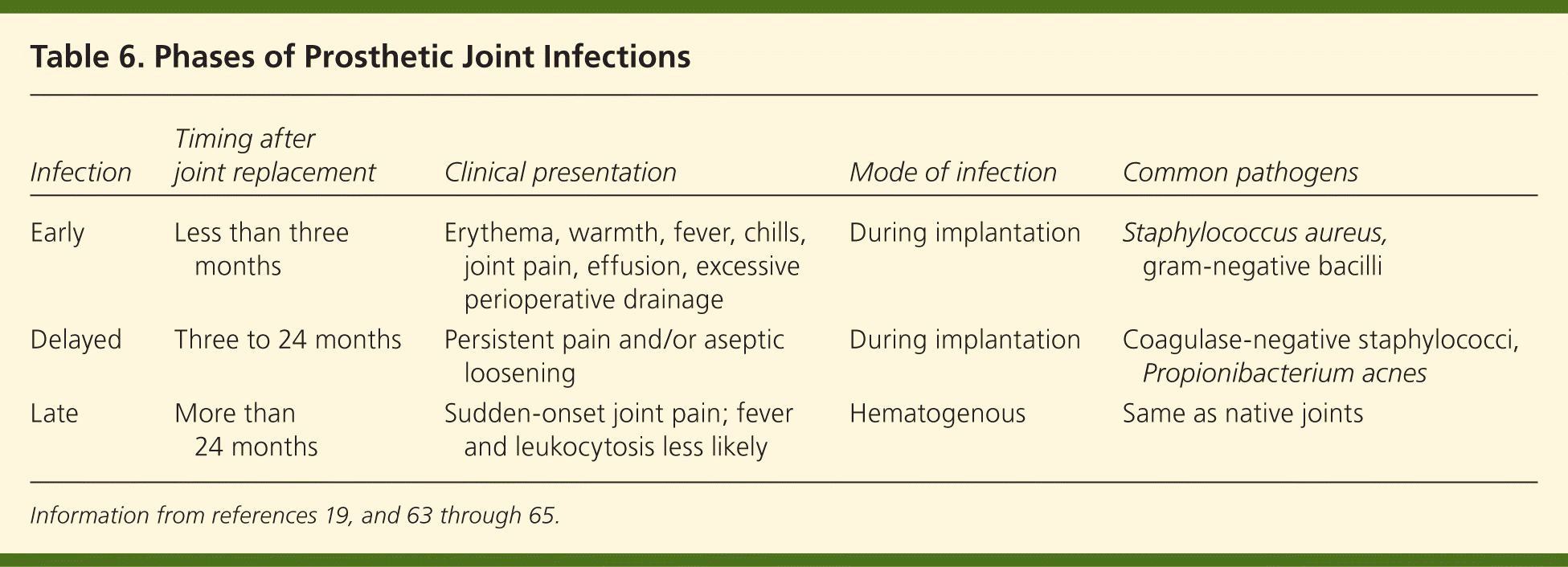
| Infection | Timing after joint replacement | Clinical presentation | Mode of infection | Common pathogens |
|---|---|---|---|---|
| Early | Less than three months | Erythema, warmth, fever, chills, joint pain, effusion, excessive perioperative drainage | During implantation | Staphylococcus aureus, gram-negative bacilli |
| Delayed | Three to 24 months | Persistent pain and/or aseptic loosening | During implantation | Coagulase-negative staphylococci, Propionibacterium acnes |
| Late | More than 24 months | Sudden-onset joint pain; fever and leukocytosis less likely | Hematogenous | Same as native joints |
A key distinction in the diagnostic workup of patients with a prosthetic joint infection is that the intraarticular WBC cutoff values for infection may be as low as 1,100 per mm3 (1.10 × 109 per L), with a neutrophil differential of greater than 64 percent.66 This low WBC count, combined with occasionally more indolent clinical presentations, can make diagnosis problematic. Diagnostic imaging modalities include fluorodeoxyglucose positron emission tomography and combined leukocyte-marrow imaging.67,68 Antimicrobial treatment must be effective against surface-adhering, biofilm-producing bacteria.58,63 Debridement, exchange, or permanent removal of the prosthesis may be necessary, depending on the clinical scenario.65 In some persons, long-term suppressive antimicrobial therapy may be warranted.63 Research is being performed on the development of antibiofilm technology to reduce the incidence of prosthetic joint infections.56,57
The use of prophylactic antibiotics for invasive dental, genitourinary, gastrointestinal, and other invasive procedures within the first two years after prosthetic joint implantation is controversial. The American Academy of Orthopaedic Surgeons recommends that physicians strongly consider antibiotic prophylaxis in patients with one or more risk factors (i.e., comorbidities, immunosuppression, and previous infection).69 However, a more recent prospective case-control study does not support this recommendation, showing no change in the incidence of prosthetic joint infections with antibiotic prophylaxis.70 The decision to use prophylactic antibiotics should be made on a case-by-case basis with input from the orthopedic surgeon and with consideration of underlying comorbidities.