
Am Fam Physician. 2012;85(4):397-399
Related: Putting Prevention into Practice
Summary of Recommendation and Evidence
The U.S. Preventive Services Task Force (USPSTF) concludes that the current evidence is insufficient to assess the balance of benefits and harms of screening for bladder cancer in asymptomatic adults (Table 1). I statement.
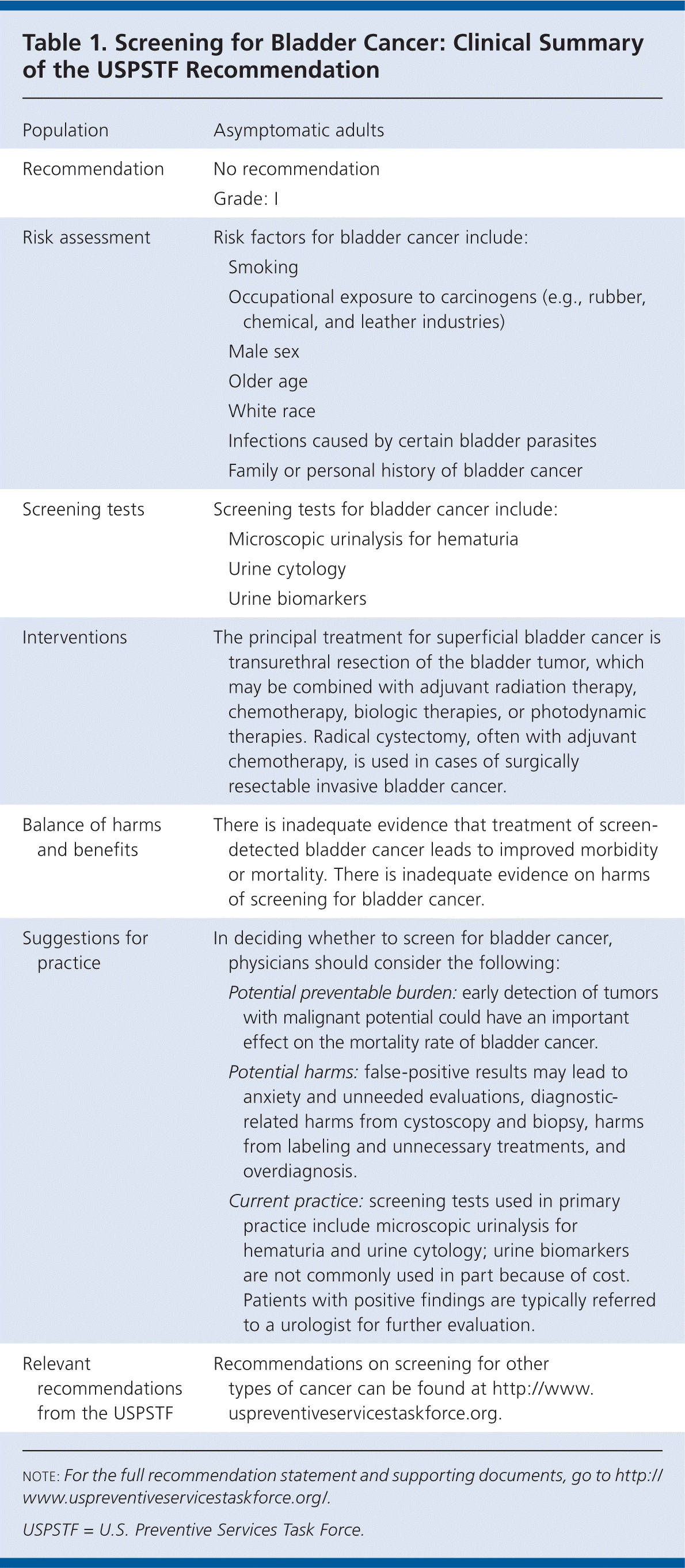
| Population | Asymptomatic adults | |
| Recommendation | No recommendation | |
| Grade: I | ||
| Risk assessment | Risk factors for bladder cancer include: | |
| Smoking | ||
| Occupational exposure to carcinogens (e.g., rubber, chemical, and leather industries) | ||
| Male sex | ||
| Older age | ||
| White race | ||
| Infections caused by certain bladder parasites | ||
| Family or personal history of bladder cancer | ||
| Screening tests | Screening tests for bladder cancer include: | |
| Microscopic urinalysis for hematuria | ||
| Urine cytology | ||
| Urine biomarkers | ||
| Interventions | The principal treatment for superficial bladder cancer is transurethral resection of the bladder tumor, which may be combined with adjuvant radiation therapy, chemotherapy, biologic therapies, or photodynamic therapies. Radical cystectomy, often with adjuvant chemotherapy, is used in cases of surgically resectable invasive bladder cancer. | |
| Balance of harms and benefits | There is inadequate evidence that treatment of screen-detected bladder cancer leads to improved morbidity or mortality. There is inadequate evidence on harms of screening for bladder cancer. | |
| Suggestions for practice | In deciding whether to screen for bladder cancer, physicians should consider the following: | |
| Potential preventable burden: early detection of tumors with malignant potential could have an important effect on the mortality rate of bladder cancer. | ||
| Potential harms: false-positive results may lead to anxiety and unneeded evaluations, diagnostic-related harms from cystoscopy and biopsy, harms from labeling and unnecessary treatments, and overdiagnosis. | ||
| Current practice: screening tests used in primary practice include microscopic urinalysis for hematuria and urine cytology; urine biomarkers are not commonly used in part because of cost. Patients with positive findings are typically referred to a urologist for further evaluation. | ||
| Relevant recommendations from the USPSTF | Recommendations on screening for other types of cancer can be found at http://www.uspreventiveservicestaskforce.org. | |
Rationale
Importance. Bladder cancer is the fourth most commonly diagnosed cancer in men and the ninth most commonly diagnosed cancer in women in the United States. It is the seventh leading cause of solid cancer–related deaths. An estimated 70,980 new cases of bladder cancer were diagnosed in the United States in 2009 (52,810 cases in men and 18,170 cases in women), and approximately 14,330 persons died of the disease (10,180 men and 4,150 women). More than 90 percent of all cases of bladder cancer are classified as transitional cell carcinomas. Most newly diagnosed transitional cell carcinomas present as superficial tumors. The stages of bladder cancer include superficial (Ta or T1) and muscle-invasive tumors. Many superficial tumors (50 to 70 percent) will recur after treatment, with a 10 to 20 percent risk of the tumor progressing to the invasive stage. One-fourth of all cases of bladder cancer and 20 to 40 percent of all invasive tumors have already metastasized to the lymph nodes at the time of diagnosis. Invasive bladder cancer is associated with a poor prognosis.
Detection. The evidence is inadequate regarding the diagnostic accuracy of potential tests (i.e., urinalysis for microscopic hematuria, urine cytology, or tests for urine biomarkers) for identifying bladder cancer in asymptomatic persons with no history of bladder cancer.
Benefits of detection and early intervention. The USPSTF found inadequate evidence that screening for bladder cancer or treatment of screen-detected bladder cancer leads to improved disease-specific or overall morbidity or mortality.
Harms of detection and early intervention. Screening may yield false-positive results. False-positive results may lead to anxiety, labeling, pain, and additional complications that result from diagnostic cystoscopy and biopsy (e.g., bladder perforation, bleeding, infection) or imaging. The USPSTF found inadequate evidence on the harms of screening for bladder cancer. Evidence on the harms associated with early treatment, which may occur more often with greater detection of cases of early-stage cancer, is also inadequate.
USPSTF assessment. The USPSTF concludes that the evidence is insufficient to determine the balance of benefits and harms of screening for bladder cancer in asymptomatic adults.
Clinical Considerations
PATIENT POPULATION
This recommendation applies to asymptomatic adults. Although adults with mild lower urinary tract symptoms (e.g., urinary frequency, hesitancy, urgency, dysuria, nocturia) are not strictly asymptomatic, these symptoms are common and are not believed to be associated with an increased risk of bladder cancer. The USPSTF considered it reasonable to include these persons in the population under consideration for screening. Adults with gross hematuria or acute changes in lower urinary tract symptoms are not included in this population.
SCREENING TESTS
Primary care–feasible screening tests for bladder cancer include identifying hematuria with a urine dipstick or microscopic urinalysis, urine cytology, and tests for urine biomarkers.
TREATMENT
After bladder cancer has been diagnosed, several factors determine treatment, including tumor grade, cancer stage (superficial versus invasive), whether the tumor is recurrent, the patient's age and overall health status, and patient and physician preferences. The principal treatment for superficial (Ta or T1) bladder cancer is transurethral resection of the bladder tumor, which may be combined with adjuvant radiation therapy, intravesical chemotherapy, immunotherapy, or photodynamic therapies. Radical cystectomy, often with adjuvant or neoadjuvant systemic chemotherapy, is used in cases of surgically resectable invasive bladder cancer.
SUGGESTIONS FOR PRACTICE
In deciding whether to screen for bladder cancer, physicians should consider the following:
Potential Preventable Burden. Bladder cancer is similar to many other types of cancer in that it is a heterogeneous condition. Approximately 70 percent of all cases of newly diagnosed transitional cell carcinomas present as superficial tumors (including in situ); some of these tumors may never progress to advanced disease. However, some cases of bladder cancer invade the muscle tissue, progress, and metastasize; treatment has limited efficacy in these cases. Early detection of tumors with malignant potential may have an important effect on the mortality rate of bladder cancer. One challenge of screening for bladder cancer is accurately identifying cases of early-stage cancer (subepithelial and in situ) with a high risk of progression. Another area of uncertainty is determining whether providing earlier, less toxic treatment (e.g., immunotherapy) with the intention of preventing symptomatic progression results in fewer overall harms to the patient than providing more toxic treatment (e.g., radical cystectomy) only to those patients who develop symptomatic or advanced tumors. Persons at increased risk of bladder cancer include those who work in the rubber, chemical, or leather industries, as well as those who smoke, are male, are older, or have a family or personal history of bladder cancer.
Potential Harms. False-positive test results may lead to anxiety and unneeded evaluations, diagnostic-related harms from cystoscopy and biopsy, harms from labeling or unnecessary treatments (e.g., transurethral resection of a bladder tumor, intravesical chemotherapy, biologic therapies), and overdiagnosis.
Current Practice. Screening tests feasible for use in primary care include urine dipstick or microscopic urinalysis for hematuria, urine cytology, and tests for urine biomarkers. Tests for urine biomarkers are not commonly used in primary care in part because of their cost, although this varies substantially. Patients with positive screening results are typically referred to a urologist for further evaluation, which may include cystoscopy (and biopsy if a tumor is found), imaging, and other studies.
