
Am Fam Physician. 2014;89(6):437-442
A more recent article on Clostridioides difficile infection is available.
Related AFP Community Blog post: Truth and Consequences of Antibiotic Overuse
Patient information: A handout on this topic is available at https://familydoctor.org/familydoctor/en/diseases-conditions/clostridium-difficile-infection.html.
Author disclosure: No relevant financial affiliations.
Clostridium difficile infection is a common cause of antibiotic-associated diarrhea. It causes no symptoms in more than one-half of infected patients, but can also cause a wide spectrum of illnesses and death. The incidence and severity have increased in recent years. The most important modifiable risk factor for C. difficile infection is antibiotic exposure; this risk is dose-related and higher with longer courses and combination therapy. C. difficile infection is also associated with older age, recent hospitalization, multiple comorbidities, use of gastric acid blockers, inflammatory bowel disease, and immunosuppression. It has become more common in younger, healthier patients in community settings. The most practical testing options are rapid testing with nucleic acid amplification or enzyme immunoassays to detect toxin, or a two-step strategy. Treatment includes discontinuing the contributing antibiotic, if possible. Mild C. difficile infection should be treated with oral metronidazole; severe infection should be treated with oral vancomycin. Fidaxomicin may be an effective alternative. Recurrences of the infection should be treated based on severity. Tapering and the pulsed-dose method of oral vancomycin therapy for second recurrences are effective. Prevention includes responsible antibiotic prescribing and vigilant handwashing. Probiotics prevent antibiotic-associated diarrhea, but are not recommended specifically for preventing C. difficile infection.
Clostridium difficile is a gram-positive anaerobic bacterium that is transmitted from person to person by the fecal-oral route. It causes 15% to 25% of cases of antibiotic-associated diarrhea.1 C. difficile infection is defined as at least three unformed stools in 24 hours and a positive stool test for C. difficile toxin or endoscopic evidence of pseudomembranous colitis.2 Overall, 7% to 26% of adults in acute care facilities are colonized with C. difficile; more than one-half of these patients are asymptomatic.2 The risk of colonization increases each day in the hospital, and symptoms usually begin within three days of colonization in symptomatic patients.2
The incidence and severity of C. difficile infection have increased. In 2005, the incidence in acute care hospitals in the United States was 84 cases per 100,000 persons, more than double the 1996 rate.3 Mortality rates increased from approximately 0.5 deaths per 100,000 persons in 1999 to approximately 2.0 deaths per 100,000 persons in 2006. Mortality rates were also higher (6.9% of those infected with C. difficile) during a hospital outbreak in Canada.2,4 The increased incidence and severity are partially due to an epidemic strain, BI/NAP1/027, which produces higher toxin levels and is highly resistant to fluoroquinolones. C. difficile infection is most prevalent in hospitalized older persons and debilitated patients, but also affects younger, healthier, community-dwelling patients. A recent study in Minnesota found that 41% of cases of C. difficile infection were community acquired.5
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Testing for Clostridium difficile infection should be performed only once during a single episode of illness because further testing does not improve diagnostic accuracy and may yield false-positive results. | C | 12, 13 |
| Vancomycin is the drug of choice for patients with severe C. difficile infection. | C | 2, 18 |
| Tapering and the pulsed-dose method of oral vancomycin therapy for second recurrences of C. difficile infection are effective. | C | 2, 22 |
| Antimicrobial stewardship programs may reduce the incidence of C. difficile infection. | C | 2 |
| Probiotics prevent antibiotic-associated diarrhea, and may reduce C. difficile–associated diarrhea in children and adults younger than 65 years. | B | 2, 34–41 |
| Recommendation | Sponsoring organization |
|---|---|
| Antibiotics should not be used for apparent viral respiratory illnesses (sinusitis, pharyngitis, bronchitis). | American Academy of Pediatrics |
| Do not use antimicrobials to treat bacteriuria in older adults unless specific urinary tract symptoms are present. | American Geriatrics Society |
Risk Factors
Risk factors for the development of C. difficile infection include age older than 64 years, recent hospitalization, antibiotic use, multiple comorbidities, use of gastric acid blockers, previous gastrointestinal surgery, inflammatory bowel disease, and immunosuppression.6,7 The risk of infection increases by approximately 2% for every year of age greater than 18 years.8,9 Infection is uncommon among children, but can occur. Patients with community-acquired C. difficile infection are younger, are more likely to be female, have fewer comorbid conditions, are less likely to develop severe infection, and are less likely to have been exposed to antibiotics.5 Antibiotic exposure is the most important modifiable risk factor. Although even single doses of prophylactic antibiotics can cause C. difficile infection, greater number of antimicrobials used, greater number of doses, and longer duration of antibiotic administration increase the risk.
Diagnosis
WHEN IS TESTING INDICATED?
Testing for C. difficile infection should be considered in patients presenting with at least three unformed stools in 24 hours.
Evidence Summary
Patients should be asked about antibiotic use in the past three months, including single perioperative doses. Symptoms vary from mild diarrhea to fulminant colitis, which can be complicated by toxic megacolon, bowel perforation, and sepsis. Less than one-half of patients with C. difficile infection have fever, abdominal discomfort, or leukocytosis. Although occult blood may be present in the stool, melena and hematochezia are uncommon.2 Ileus is a rare presentation of C. difficile infection.10 Guidelines from the American College of Gastroenterology recommend testing all patients with inflammatory bowel disease hospitalized with a disease flare-up.11
HOW IS IT DIAGNOSED?
The diagnosis of C. difficile infection is primarily clinical, although many different tests are available. Clinicians should become familiar with the testing approach used by their laboratory. For a single episode of illness, testing should be performed only once because further testing does not improve diagnostic accuracy and may yield false-positive results.12,13
Evidence Summary
Many patients are colonized with C. difficile, but signs and symptoms occur only when toxin is produced. To reduce false-positive results, appropriate selection of patients for testing is important. One study demonstrated that many patients inappropriately tested for C. difficile infection did not have diarrhea or had recently used laxatives.14
Enzyme immunoassay is widely used as a rapid test to detect toxins produced by C. difficile. Its specificity is high (83% to 98%), but its sensitivity is lower (75% to 95%), because a low level of toxin can lead to false-negative results.11 Consequently, many institutions have switched to the use of more sensitive and specific nucleic acid amplification testing, which includes polymerase chain reaction, as recommended by the American College of Gastroenterology.11 Recent studies have shown a significant increase in population-based incidence rates of C. difficile infection when laboratories transition from a one-step strategy using enzyme immunoassay to using nucleic acid amplification testing.15 This approach raises concerns that the increase is due to detection of less severe or subclinical cases, as well as carriers who have diarrhea from other causes. Yet, rapid identification by nucleic acid amplification testing allows for earlier isolation and treatment of patients with C. difficile infection, as well as eliminates the need for repeat testing.
An alternative to a one-step approach using nucleic acid amplification testing or enzyme immunoassay is a multistep protocol in which the first step is detection of the glutamate dehydrogenase antigen, which is produced by all C. difficile isolates.16 If this rapid and sensitive test is positive, samples should then undergo analysis to verify toxin production (with one or more of the previously mentioned tests). Further studies are needed to clarify the testing strategy that leads to the most favorable patient outcomes.11,16,17
Testing for cure should be avoided in asymptomatic patients because the toxin may be produced after clinical disease has resolved.2
Treatment
WHAT IS THE BEST APPROACH TO DRUG SELECTION FOR THE FIRST EPISODE?
Treatment includes discontinuing the contributing antibiotic, if it is no longer indicated or an alternative is available. The Society for Healthcare Epidemiology of America and the Infectious Diseases Society of America recommend oral metronidazole (Flagyl) for mild cases of C. difficile infection. Oral vancomycin is the preferred agent for severe infection.2,18
Evidence Summary
Compared with metronidazole, vancomycin capsules are expensive, but the generic intravenous formulation may be compounded into a less expensive oral solution.19
For complicated C. difficile infection with ileus, higher dosages of vancomycin (500 mg four times per day) are recommended, although evidence supporting higher dosages is scant.2 Intravenous metronidazole combined with oral vancomycin may be necessary for severe infection, and vancomycin enemas can also be used.
A randomized, placebo-controlled trial compared oral vancomycin (125 mg four times per day) with oral metronidazole (250 mg four times per day), stratifying patients according to infection severity.18 Severe infection was defined as meeting two of the following criteria: age older than 60 years, temperature higher than 100.9°F (38.3°C), albumin level less than 2.5 g per dL (25 g per L), or white blood cell count greater than 15,000 per μL (15 × 109 per L) within 48 hours of enrollment. Severe infection also included endoscopic evidence of pseudomembranous colitis or intensive care unit treatment. Metronidazole and vancomycin were equally effective for mild infection, whereas vancomycin was superior for severe infection.
Fidaxomicin (Dificid) has a narrow spectrum of activity, which may preserve beneficial gastrointestinal flora, and has high bactericidal activity against C. difficile, including the BI/NAP1/027 strain.20 A randomized trial compared fidaxomicin (200 mg twice per day) with vancomycin (125 mg four times per day) given orally for 10 days.21 Fidaxomicin was noninferior to vancomycin for clinical cure (88.2% and 89.8%, respectively). Fidaxomicin produced a significantly lower recurrence rate overall (15.4% vs. 25.3%, respectively), although the recurrence rates for the BI/NAP1/027 strain were similar. Fidaxomicin appears to be effective for the treatment of C. difficile infection, but more studies are needed to define its role in therapy.
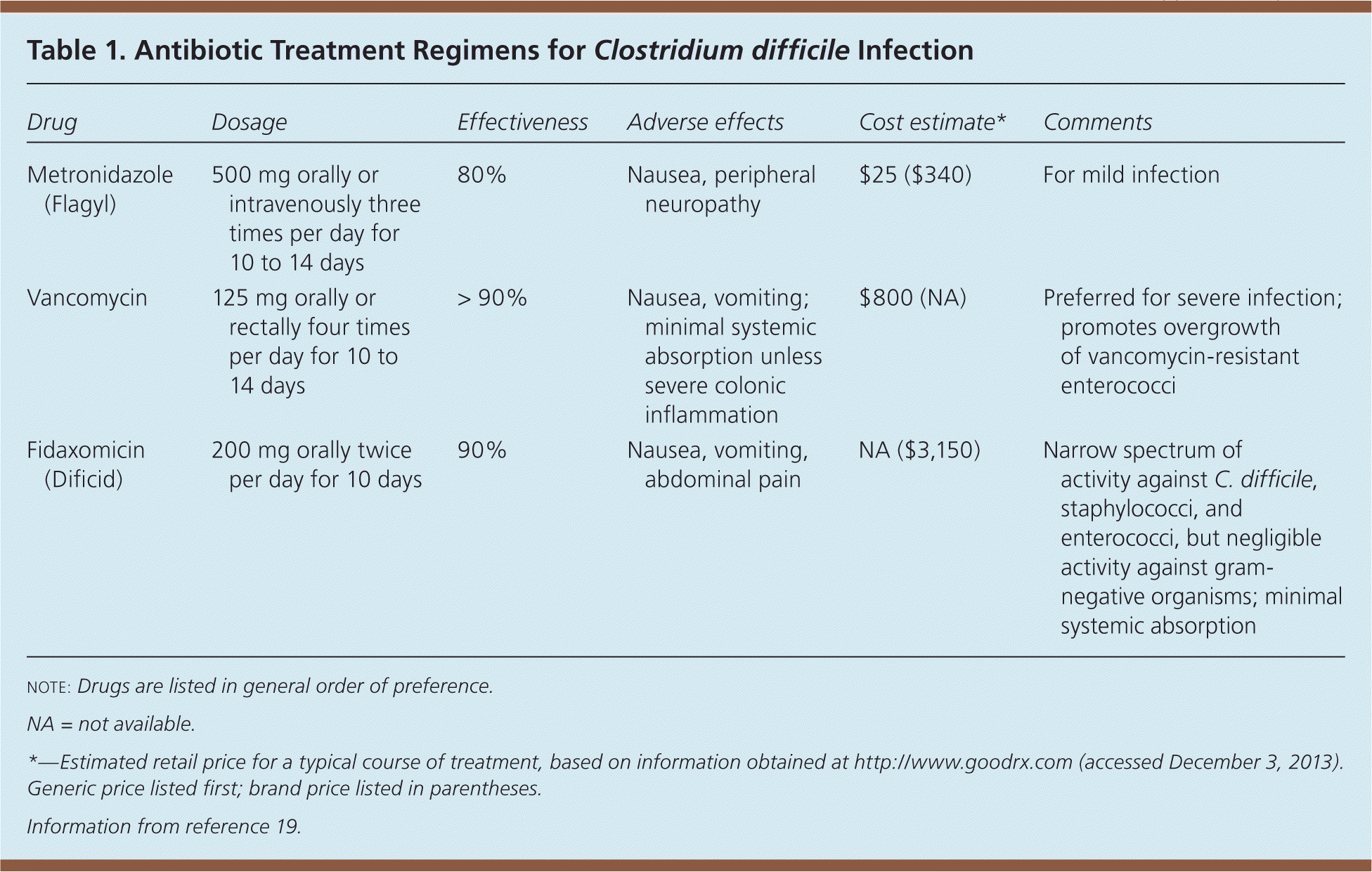
| Drug | Dosage | Effectiveness | Adverse effects | Cost estimate* | Comments |
|---|---|---|---|---|---|
| Metronidazole (Flagyl) | 500 mg orally or intravenously three times per day for 10 to 14 days | 80% | Nausea, peripheral neuropathy | $25 ($340) | For mild infection |
| Vancomycin | 125 mg orally or rectally four times per day for 10 to 14 days | > 90% | Nausea, vomiting; minimal systemic absorption unless severe colonic inflammation | $800 (NA) | Preferred for severe infection; promotes overgrowth of vancomycin-resistant enterococci |
| Fidaxomicin (Dificid) | 200 mg orally twice per day for 10 days | 90% | Nausea, vomiting, abdominal pain | NA ($3,150) | Narrow spectrum of activity against C. difficile, staphylococci, and enterococci, but negligible activity against gram-negative organisms; minimal systemic absorption |
HOW SHOULD RECURRENT INFECTION BE TREATED?
An initial recurrence should be treated with metronidazole or vancomycin if the recurrent infection is mild, but vancomycin is indicated for severe infection.18 Tapering and the pulsed-dose method of oral vancomycin therapy for second recurrences are effective.22 Intestinal microbial transplantation also resolves symptoms in most patients with recurrent infection.
Evidence Summary
Overall, 20% to 30% of patients with C. difficile infection experience a recurrence of the infection within 60 days. Similar recurrence rates are reported with vancomycin and metronidazole. A second course of either drug for recurrent infection does not increase the risk of an additional episode.22,23 Metronidazole should not be used for subsequent recurrences because of the risk of neurotoxicity.2 A typical dosing regimen of oral vancomycin includes 125 mg four times per day for 10 to 14 days, 125 mg two times per day for one week, 125 mg per day for one week, and then 125 mg every two or three days for two to eight weeks.
Intestinal microbial transplantation, or fecal bacteriotherapy, infuses stool from a healthy donor into the intestinal tract of a patient who has had recurrent C. difficile infection. A systematic review found that fecal bacteriotherapy prevented recurrent infection in 92% of 317 patients in 27 case studies.26 Results varied by technique, and no major adverse events were noted. A follow-up study of 77 patients over an average of 17 months found that 91% achieved resolution of symptoms within 90 days.27 A randomized trial of 41 patients with at least one relapse found that fecal infusion achieved cure in 81% of patients, compared with 31% in those receiving vancomycin alone and 23% in those receiving vancomycin plus bowel lavage.28
Prevention
HOW CAN CLINICIANS ADJUST ANTIBIOTIC USE TO PREVENT C. DIFFICILE INFECTION?
Minimizing the frequency and duration of antimicrobial therapy and the number of antimicrobial agents prescribed, as well as implementing an antimicrobial stewardship program, are recommended.2
Evidence Summary
The Society for Healthcare Epidemiology of America and the Infectious Diseases Society of America guidelines indicate that restricting cephalosporin and clindamycin use, except for surgical prophylaxis, may prevent C. difficile infection.2
DO HAND HYGIENE AND CONTACT PRECAUTIONS PREVENT C. DIFFICILE INFECTION?
Handwashing with soap and water or chlorhexidine and barrier precautions should be used routinely in patients with C. difficile infection to prevent transmission.
Evidence Summary
Health care workers and visitors who come into contact with persons who have C. difficile infection should wash their hands.2 Handwashing with soap and water is more effective than alcohol-based hand sanitizer and antiseptic wipes, because alcohol does not kill C. difficile spores.2,29,30 Antibacterial soap and chlorhexidine are also effective.31 Gloves, disposable thermometers, and sporicidal disinfectants should be used.2 Gown use and isolation of contaminated patients are recommended.2,32
Contact precautions should be considered for patients with a history of C. difficile infection because skin contamination and shedding can continue for weeks after diarrhea resolves.33 There are few data regarding the testing and treatment of asymptomatic C. difficile infection, but this practice is common.
DO PROBIOTICS PREVENT C. DIFFICILE INFECTION?
The Society for Healthcare Epidemiology of America and the Infectious Diseases Society of America do not recommend probiotics to reduce the risk of primary C. difficile infection.2 However, recent randomized trials and meta-analyses found that probiotics reduced antibiotic-associated diarrhea and may reduce C. difficile–associated diarrhea in children and adults younger than 65 years, both as inpatients and outpatients.34–40
Evidence Summary
One randomized trial of 135 patients evaluated a probiotic drink containing Lactobacillus casei, Lactobacillus bulgaricus, and Streptococcus thermophilus. After four weeks, none of the patients who took probiotics developed C. difficile infection compared with 17% of the patients who took placebo (number needed to treat [NNT] = 6).34
Another trial of 255 adults taking antibiotics compared two capsules of a probiotic containing 50 billion colony-forming units of Lactobacillus acidophilus plus L. casei, one capsule of the probiotic, and placebo.35 Patients began probiotics or placebo within 36 hours of starting antibiotics and continued until five days after antibiotic cessation. C. difficile infection incidence three weeks after completion of the intervention was 1.2% in the high-dose probiotic group (NNT = 4), 9.4% in the low-dose probiotic group (NNT = 7), and 23.8% in the placebo group. No adverse events were noted in either trial.34,35
A meta-analysis of 63 randomized controlled trials found a statistically significant reduction in antibiotic-associated diarrhea in patients taking probiotics (NNT = 13).36 However, this analysis could not determine if probiotics prevented diarrhea specifically caused by C. difficile infection. Another meta-analysis of 20 randomized controlled trials found that probiotics decreased the risk of C. difficile–associated diarrhea by 66% in adults and children. There was no difference between probiotic species used, and trials using multiple species had more robust results compared with those using one species, although both showed statistically significant improvement. Adverse events were less common in the probiotic group than in the group taking placebo.41
A third meta-analysis of 84 trials examined the effect of probiotics in preventing many gastrointestinal diseases that included C. difficile. A significant benefit of probiotic use occurred in 37% of the studies, whereas 63% of the studies found no benefit to probiotic use. A pooled estimate of the effectiveness of probiotics found a significant 42% risk reduction in the prevention or treatment of gastrointestinal disease. Eight species of probiotics were effective, but no difference was noted between single species and multiple species probiotic formulations. Infants, children, and adults all benefited from therapy, and longer treatment durations (nine to 240 weeks) were more effective than shorter treatment durations (three to four weeks).37
A Cochrane review of 23 trials of children and adults, both inpatients and outpatients, taking antibiotics found that probiotics reduced the risk of C. difficile–associated diarrhea by 64% (NNT = 29). Probiotics also reduced the risk of developing adverse effects, such as taste disturbance, abdominal cramping, flatulence, nausea, and fever, by 20%. No significant difference in the incidence of C. difficile infection was noted.38 Another Cochrane review of 15 studies evaluated the prevention of antibiotic-associated diarrhea in hospitalized and ambulatory children taking antibiotics. Pooled results showed probiotics reduced the incidence of diarrhea by 48% (NNT = 7 with high probiotic doses). There was significant heterogeneity in probiotic strain, dose, duration, and study quality.39
Effectiveness of a high-dose Lactobacilli/Bifidobacteria probiotic formulation in preventing antibiotic-associated diarrhea (including diarrhea caused by C. difficile) was assessed in hospitalized adults older than 65 years in a recent randomized, placebo-controlled trial (N = 2,981). The incidence of C. difficile–associated diarrhea was lower in the treatment group (0.8% compared with 1.2% in the placebo group); however, this was not statistically significant. Of note, stool samples were not obtained in about 40% of participants because of short duration of diarrhea, which may have missed some cases of C. difficile infection.40
Data Sources: A PubMed search was completed in Clinical Queries using the key terms Clostridium difficile; diagnosis; treatment; risk factors; and prevention and control, or tertiary or secondary prevention. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. Limits included English-language articles about human subjects. Also searched were the Agency for Healthcare Research and Quality evidence reports, the Cochrane database, Essential Evidence Plus, the Institute for Clinical Systems Improvement, and the National Guideline Clearinghouse database. Search date: December 23, 2013.
