
Am Fam Physician. 2014;90(2):91-96
A more recent article on fever of unknown origin in adults is available.
Patient information: See related handout on fever of unknown origin in adults, written by the authors of this article.
Author disclosure: No relevant financial affiliations.
Fever of unknown origin has been described as a febrile illness (temperature of 101°F [38.3°C] or higher) for three weeks or longer without an etiology despite a one-week inpatient evaluation. A more recent qualitative definition requires only a reasonable diagnostic evaluation. Although there are more than 200 diseases in the differential diagnosis, most cases in adults are limited to several dozen possible causes. Fever of unknown origin is more often an atypical presentation of a common disease rather than an unusual disease. The most common subgroups in the differential are infection, malignancy, noninfectious inflammatory diseases, and miscellaneous. Clinicians should perform a comprehensive history and examination to look for potentially diagnostic clues to guide the initial evaluation. If there are no potentially diagnostic clues, the patient should undergo a minimum diagnostic workup, including a complete blood count, chest radiography, urinalysis and culture, electrolyte panel, liver enzymes, erythrocyte sedimentation rate, and C-reactive protein level testing. Further testing should include blood cultures, lactate dehydrogenase, creatine kinase, rheumatoid factor, and antinuclear antibodies. Human immunodeficiency virus and appropriate region-specific serologic testing (e.g., cytomegalovirus, Epstein-Barr virus, tuberculosis) and abdominal and pelvic ultrasonography or computed tomography are commonly performed. If the diagnosis remains elusive, 18F fluorodeoxyglucose positron emission tomography plus computed tomography may help guide the clinician toward tissue biopsy. Empiric antibiotics or steroids are generally discouraged in patients with fever of unknown origin.
Fever of unknown origin (FUO) in adults is one of the most vexing clinical conditions for clinicians and patients. There are no published guidelines, nor is there a recommended standard approach to the diagnosis. The definition of what constitutes FUO remains controversial.1,2 FUO was first described in a 1961 case series as prolonged febrile illness (temperature of 101°F [38.3°C] or higher) for three weeks or longer that did not have an established etiology despite a one-week inpatient evaluation.3,4 The arbitrarily defined three weeks allowed most acute, self-limited illnesses to resolve, as well as sufficient time to complete the initial investigation.5,6
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| A comprehensive history and physical examination should be performed if there are no localizing signs and symptoms in patients with prolonged febrile illness. | C | 15, 17–21 |
| Potentially diagnostic clues should be sought during the history and physical examination to guide further evaluation of prolonged febrile illness. | C | 15–17 |
| In patients with a prolonged febrile illness, a minimum diagnostic workup should be performed before classifying the disease process as a fever of unknown origin. | C | 1, 2, 4–7, 15–20, 27 |
| Erythrocyte sedimentation rate and C-reactive protein levels should be measured in the initial workup of a patient who has prolonged febrile illness without a clear source. | C | 5, 15, 28, 29 |
| In patients who have a fever of unknown origin with an elevated erythrocyte sedimentation rate and/or C-reactive protein levels, and who have not received a diagnosis after initial evaluation, 18F fluorodeoxyglucose positron emission tomography scan with or without computed tomography may be useful in reaching a diagnosis. | C | 15, 37–40 |
| If noninvasive diagnostic tests are unrevealing, then the invasive test of choice is a tissue biopsy because of the relatively high diagnostic yield. Depending on clinical clues, this may include liver, lymph node, temporal artery, or bone marrow biopsy. | C | 2, 3, 5, 15, 19, 22, 27, 41 |
FUO was further defined in 1991, suggesting that the minimum evaluation be changed to at least three outpatient visits or three days in inpatient care.7 Others have proposed shorter lengths of time (e.g., two weeks, because today's patients present earlier and receive a diagnosis more quickly).8,9 A retrospective review of 226 hospitalized febrile patients examined the timing of diagnosis from initial visit for fever through the end of hospitalization. There was no difference in types of diagnoses for those who met the strict 1991 definition compared with those who received a diagnosis in less than three weeks.10 Therefore, FUO may be assumed when no reasonable diagnosis is reached after an appropriate inpatient or outpatient investigation.2,6,10–17 Table 1 compares the evolution of the definition of FUO.2,3,6,7,10–17
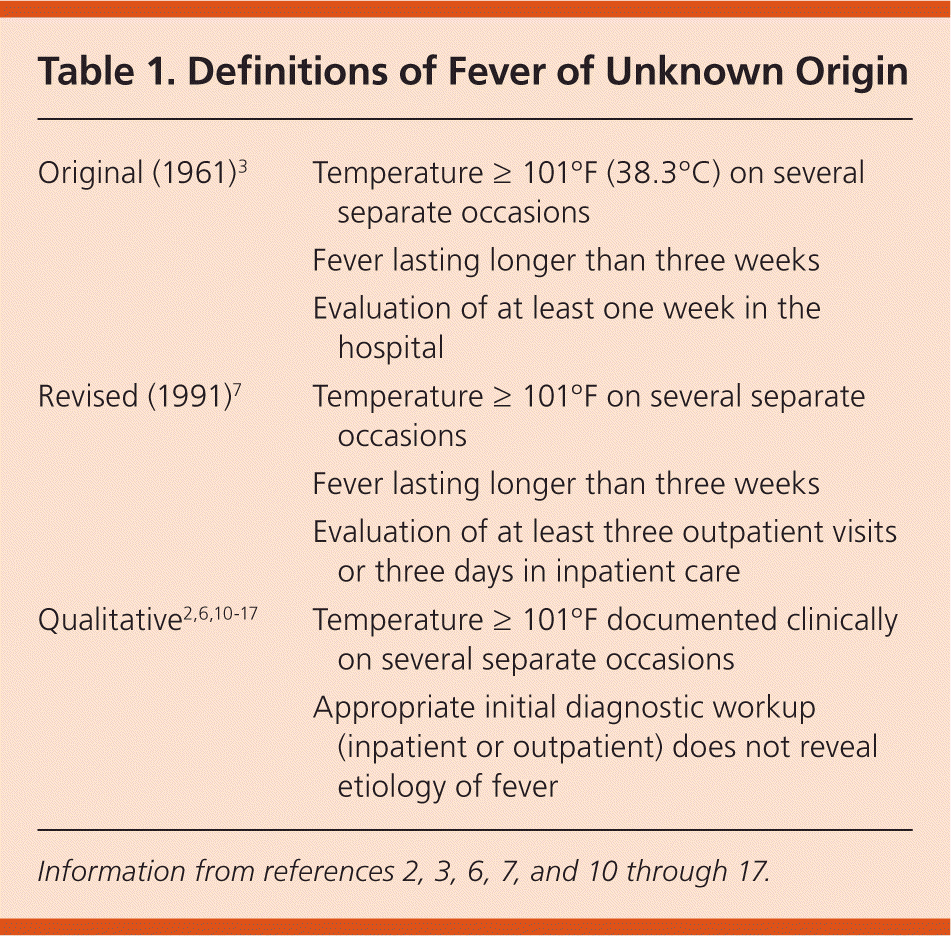
| Original (1961)3 | Temperature ≥ 101°F (38.3°C) on several separate occasions |
| Fever lasting longer than three weeks | |
| Evaluation of at least one week in the hospital | |
| Revised (1991)7 | Temperature ≥ 101°F on several separate occasions |
| Fever lasting longer than three weeks | |
| Evaluation of at least three outpatient visits or three days in inpatient care | |
| Qualitative2,6,10–17 | Temperature ≥ 101°F documented clinically on several separate occasions |
| Appropriate initial diagnostic workup (inpatient or outpatient) does not reveal etiology of fever |
Differential Diagnosis
The etiologies of FUO have changed over time because of shifting disease patterns and new diagnostic techniques.14 There are more than 200 diseases in the differential diagnosis.4,15,17 In multiple case series, however, the etiology of FUO is limited to several dozen causes, and patients often have an atypical presentation of a common disease.2,6,18
Common causes of FUO are listed in Table 2.6,15–23 Typical subgroups used in the differential for classical FUO are infection (20% to 40%), malignancy (20% to 30%), noninfectious inflammatory diseases (10% to 30%), miscellaneous (10% to 20%), and undiagnosed (up to 50%).1,4–6,14–18,22–24 Noninfectious inflammatory diseases commonly include connective tissue diseases, vasculitides, and granulomatous diseases.16,17 In developed countries, the noninfectious inflammatory diseases and undiagnosed groups comprise a higher proportion of FUO cases.5,10,15,17 Underdeveloped countries have higher rates of infection and neoplasm.6,24 Drug fever is implicated in 1% to 3% of FUO cases16 (Table 320,21,25,26 ).

| Subgroup | Cause | |
|---|---|---|
| Infection (20% to 40%) | Bacterial | |
| Abdominal or pelvic abscesses | ||
| Dental abscesses | ||
| Endocarditis | ||
| Sinusitis | ||
| Tuberculosis (especially extrapulmonary/disseminated) | ||
| Urinary tract infection | ||
| Viral | ||
| Cytomegalovirus | ||
| Epstein-Barr virus | ||
| Malignancy (20% to 30%) | Colorectal cancer | |
| Leukemia | ||
| Lymphoma (Hodgkin and non-Hodgkin) | ||
| Noninfectious inflammatory disease (10% to 30%) | Connective tissue diseases | |
| Adult Still disease | ||
| Rheumatoid arthritis | ||
| Systemic lupus erythematosus | ||
| Granulomatous disease | ||
| Crohn disease | ||
| Sarcoidosis | ||
| Vasculitis syndromes | ||
| Giant cell arteritis | ||
| Polymyalgia rheumatica/temporal arteritis | ||
| Miscellaneous (10% to 20%) | Drug-induced | |
| Factitious fever | ||
| Thromboembolic disease | ||
| Thyroiditis | ||
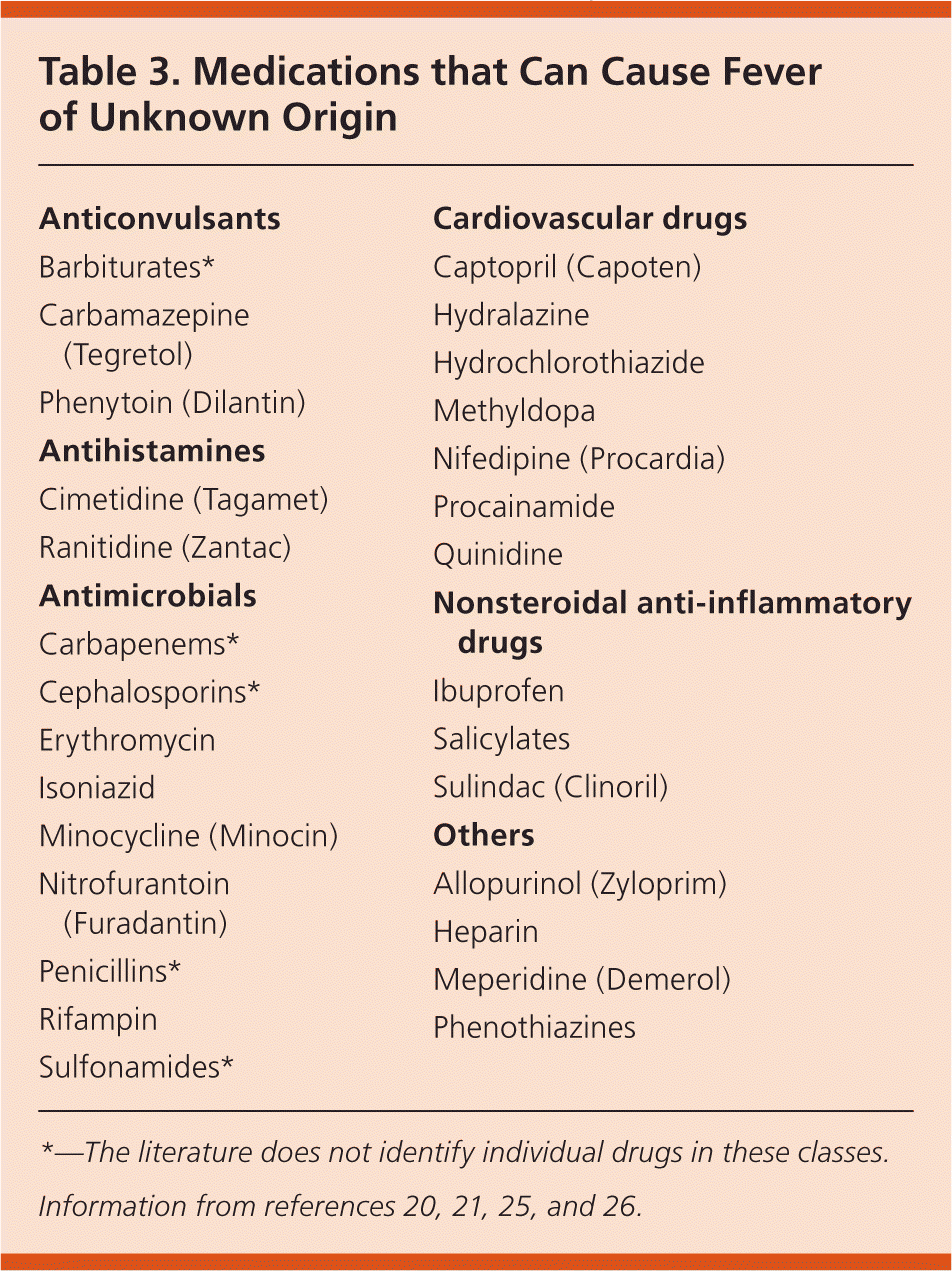
| Anticonvulsants |
| Barbiturates* |
| Carbamazepine (Tegretol) |
| Phenytoin (Dilantin) |
| Antihistamines |
| Cimetidine (Tagamet) |
| Ranitidine (Zantac) |
| Antimicrobials |
| Carbapenems* |
| Cephalosporins* |
| Erythromycin |
| Isoniazid |
| Minocycline (Minocin) |
| Nitrofurantoin (Furadantin) |
| Penicillins* |
| Rifampin |
| Sulfonamides* |
| Cardiovascular drugs |
| Captopril (Capoten) |
| Hydralazine |
| Hydrochlorothiazide |
| Methyldopa |
| Nifedipine (Procardia) |
| Procainamide |
| Quinidine |
| Nonsteroidal anti-inflammatory drugs |
| Ibuprofen |
| Salicylates |
| Sulindac (Clinoril) |
| Others |
| Allopurinol (Zyloprim) |
| Heparin |
| Meperidine (Demerol) |
| Phenothiazines |
From Prolonged Febrile Illness to FUO
Because there are no guidelines to the approach of the febrile patient, most evaluation recommendations are based on expert opinion.17 On initial presentation, most clinicians perform a history and physical examination in pursuit of an infection. When there are no clear localizing signs or symptoms, clinicians should expand on the patient's symptoms and historical information, looking for potentially diagnostic clues to guide the evaluation (Table 4).17–20,25,27 This is a continuous, iterative process.19–21 Potentially diagnostic clues lead to a diagnosis in 62% of patients, although clues can be misleading because they are found in 97% of patients.15–17

| Clues | Possible diagnoses | |
|---|---|---|
| Historical | ||
| Exposures | ||
| Fresh water exposure | Leptospirosis | |
| Living conditions (e.g., homeless shelter) | Tuberculosis | |
| Occupational exposures/sick contacts (e.g., with hospitalized patients, children) | Cytomegalovirus, Epstein-Barr virus, tuberculosis | |
| Pets, wild animals | Brucellosis | |
| Recent travel, especially to areas with endemic diseases (domestic and abroad) | Region specific (e.g., Q fever for parts of Europe) | |
| Family history | ||
| Hereditary febrile conditions | Familial Mediterranean fever | |
| Medical history | ||
| Abdominal disorders | Alcoholic hepatitis, cirrhosis, Crohn disease | |
| History of transfusions | Hepatitis B or C, HIV | |
| Malignancy | Metastatic disease | |
| Psychiatric illness | Factitious fever | |
| Recent hospitalization | Nosocomial infection | |
| Risk-taking behaviors | ||
| Intravenous drug abuse | Abscess, endocarditis, osteomyelitis | |
| Sexually transmitted infection exposure | HIV | |
| Surgical history | ||
| Presence of prostheses | Osteomyelitis | |
| Physical | ||
| Characteristic rashes (e.g., erythema multiforme, petechiae) | Adenovirus, herpes simplex virus, HIV, meningococcemia, tick-borne illness | |
| Conjunctivitis or uveitis | Adult Still disease, leptospirosis, systemic lupus erythematosus | |
| Hepato- or splenomegaly; palpable abdominal masses | Alcoholic liver disease, carcinoma, cytomegalovirus, Epstein-Barr virus, leukemia, lymphoma | |
| Joint swelling or pain with movement | Inflammatory bowel disease, Lyme disease, systemic lupus erythematosus | |
| Lymphadenopathy | Cat-scratch disease, cytomegalovirus, Epstein-Barr virus, HIV | |
If no potentially diagnostic clues are found, a minimum diagnostic workup should be performed. Infections predominate early in FUO diagnoses, and the longer FUO remains undiagnosed, the less likely it is caused by an infection.27 After infections, the etiology of FUO transitions to noninfectious inflammatory diseases and malignancies, which can guide subsequent testing. Figure 1 outlines a diagnostic approach to patients with prolonged febrile illness and FUO.1,2,4–7,15–20,23,27
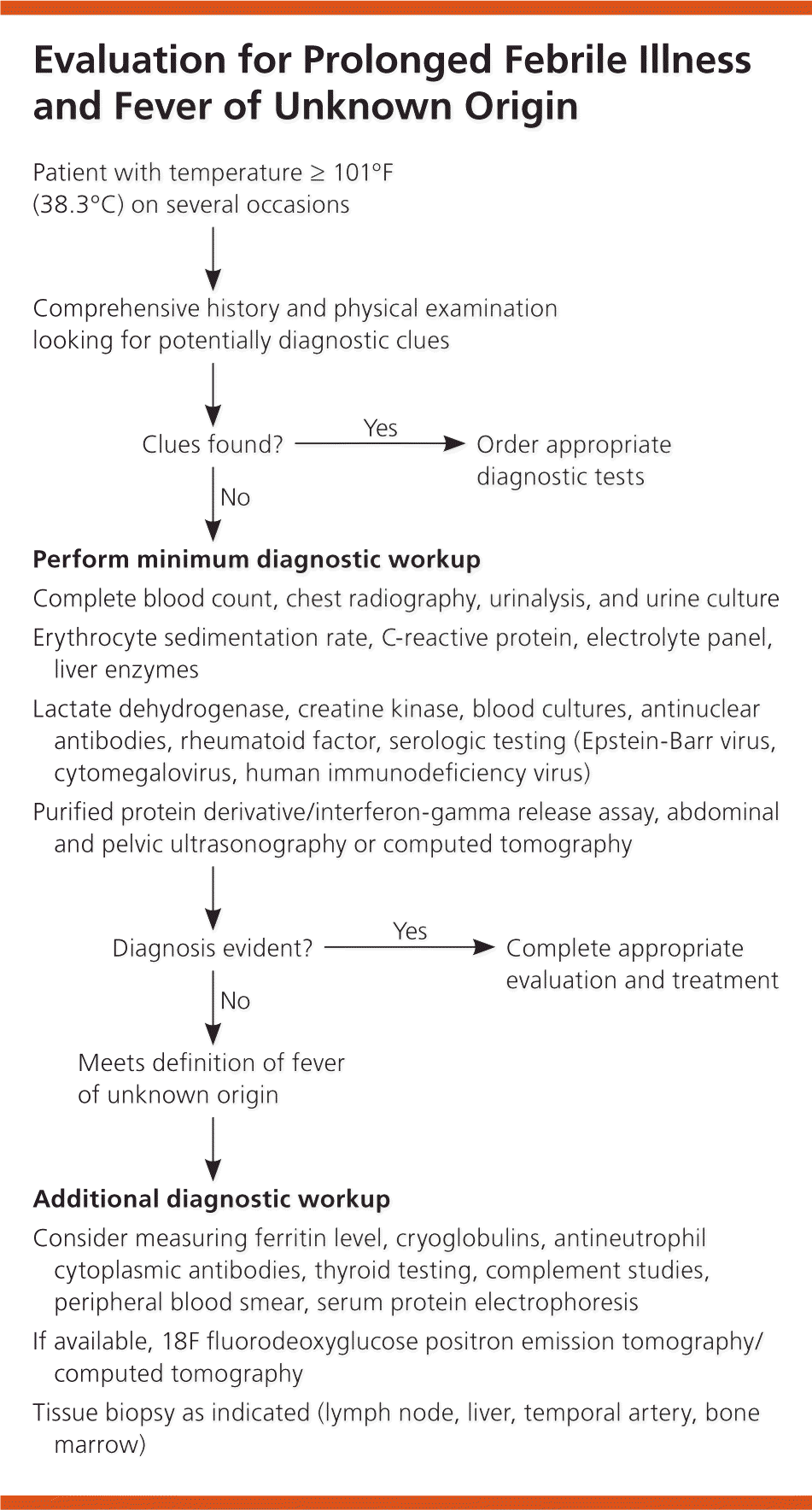
Hospitalization may be considered at any time during the evaluation, especially if the patient exhibits signs of a critical illness. Approximately 12% to 35% of patients die from an FUO-related cause (generally infection or malignancy), yet of those whose conditions remain undiagnosed, most recover or have a benign course with a good prognosis.5,22
COMMON INFECTIONS
At the initial encounter, testing for common infections should include a complete blood count with differential, electrolyte panel, liver enzymes, urinalysis with culture, blood culture, and chest radiography. If there is no clear source of infection, then further testing should follow.
Erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are nonspecific acute-phase reactants that are routinely part of the evaluation of febrile patients.5,28 An extremely elevated ESR (100 mm per hour or greater) suggests etiologies such as abdominal or pelvic abscess, osteomyelitis, and endocarditis. However, ESR does not help discriminate between active autoimmune disease and infection, and malignancies and noninfectious inflammatory diseases can cause an elevated ESR and CRP level. In one review, an ESR of 100 mm per hour or greater had a high specificity for malignancy (96%) and infection (97%), and its positive predictive value was 90%.29 A normal ESR has a high negative predictive value for temporal arteritis.28,30 An ESR that is not elevated has no diagnostic value and does not rule out neoplastic or other disorders.27 CRP level is a sensitive marker for infection and inflammation, but it is not sensitive enough to discriminate between disease processes.28 However, a more recent prospective study found that the chance of establishing a diagnosis was higher in patients who had an elevated CRP level and ESR.15
Procalcitonin is a newer marker specific for bacterial infection. In multiple studies, procalcitonin has been shown to have a specificity ranging from 70% to 98%, with a higher specificity for bacterial infection than other markers.28,31,32 It may be helpful in distinguishing between fevers with a bacterial cause vs. noninfectious inflammatory diseases, but its role in the workup of FUO is currently undefined.28,32
MALIGNANCIES AND NONINFECTIOUS INFLAMMATORY DISEASES
If the diagnosis remains elusive, tests targeting malignancies and noninfectious inflammatory diseases should be considered. Elevated lactate dehydrogenase levels can be indicative of infectious and malignant causes of FUO, including malaria, lymphoma, and leukemia.15,21 Measurement of ferritin levels may also be helpful.33 An elevated ferritin level in prolonged febrile illness may indicate malignancy (especially myeloproliferative disorders) and other noninfectious inflammatory diseases, such as systemic lupus erythematosus or temporal arteritis.21,33 One study established a ferritin level of 561 ng per mL (1,261 pmol per L) as the optimal cutoff value to predict that FUO was due to a noninfectious cause.22 Extreme elevation of ferritin levels (greater than 1,000 ng per mL [2,247 pmol per L]) can point to adult Still disease.34 Infection is the most common reason ESR is extremely elevated, but if there is no evidence of infectious causes, clinicians should consider malignancy, renal disease, and inflammatory disorders if the ESR is 100 mm per hour or greater.29
Testing for antinuclear antibodies, rheumatoid factor, human immunodeficiency virus, Epstein-Barr virus, cytomegalovirus, purified protein derivative (or interferon-gamma release assay), and antineutrophil cytoplasmic antibodies, as well as measurement of the creatine kinase level, can suggest other infectious sources and common noninfectious inflammatory disease etiologies, such as systemic lupus erythematosus, rheumatoid arthritis, and vasculitides. Previous testing (ESR, complete blood count, electrolyte panel, chest radiography, urinalysis, blood culture) may be repeated periodically to evaluate for trends as the illness evolves. Age-appropriate or potentially diagnostic clue–guided cancer screening should be performed (e.g., colonoscopy in patients 50 years or older).
Abdominal and pelvic ultrasonography are often recommended in the initial workup because of availability, low cost, and lack of radiation exposure.15 After the initial evaluation is complete and if there is no diagnosis, the patient is considered to have FUO, and a secondary evaluation should be considered.
Secondary Evaluation
Several diagnostic algorithms have been suggested for FUO, but few are supported by evidence from prospective studies.17 Region-specific serologic tests, more advanced radiologic studies, and more invasive diagnostic procedures can be guided by potentially diagnostic clues. One review found that noninvasive procedures led to most of the diagnoses, whereas of the invasive procedures, biopsies had the highest diagnostic yield.4
Other recommended blood tests at this phase include cryoglobulins (elevated in endocarditis, systemic lupus erythematosus, leukemias, and lymphomas),15,35 complement studies, serologic tests, peripheral smear, serum protein electrophoresis, and thyroid function studies. Note that serologic tests are helpful only if there are potentially diagnostic clues and if the patient lives in or has visited an area where the suspected disease is prevalent.15
IMAGING STUDIES
Chest, abdominal, or pelvic computed tomography (CT) may be useful in the secondary evaluation. In one study of patients with FUO, chest and abdominal CT had high sensitivity (82% and 92%, respectively) and were recommended if the initial evaluation was unrevealing.15 CT specificity ranged from 60% to 70%, consistent with other case series.15,16 Echocardiography is recommended if there are clinical indications of endocarditis.5,20 Venous Doppler ultrasonography is indicated for suspected thromboembolism.20 Magnetic resonance imaging of the aortic arch and great vessels of the neck was shown to be helpful when vasculitis was suspected.36
Nuclear imaging studies are noninvasive, image the whole body, and can localize a potential infectious or inflammatory cause for FUO.5,14,19,37–40 Recently, 18F fluorodeoxyglucose positron emission tomography technology has been evaluated for guiding further invasive testing, especially in patients who have an elevated ESR or CRP level.14,37 The 18F fluorodeoxyglucose is taken up by inflammatory and cancer cells because of their high rate of glucolysis.14,18,37 Several studies examining this method in patients with FUO found diagnostic yields ranging from 16% to 69%,15,37,38 with a high positive predictive value (93%) and negative predictive value (100%).39,40 A hybrid of CT and 18F fluorodeoxyglucose positron emission tomography has a higher diagnostic yield (sensitivity of 56% to 100%; specificity of 75% to 81%18). The 18F fluorodeoxyglucose has better uptake and is cleared more rapidly than older modalities (e.g., gallium Ga 67 citrate), but it is costly and not widely available.14
BIOPSIES
Liver, lymph node, or temporal artery biopsy may help establish a definitive diagnosis.3,19 A prospective study of 192 patients found that biopsies produced up to a 35% diagnostic yield (about 10% to 35%), especially if performed later in the evaluation when infection is less likely, and malignancies and noninfectious inflammatory diseases are more common.2 Liver biopsy, with a diagnostic yield between 14% and 17%,5,19 can reveal granulomatous hepatitis and determine its cause, which could be infectious, inflammatory, or neoplastic processes.22,27 Lymph node biopsy is most useful in diagnosing lymphoma, infectious diseases, and granulomatous diseases.19,27 In patients 55 years or older, temporal arteritis causes more than 15% of cases of FUO, so biopsy should be considered.5,15,18
Bone marrow biopsy is diagnostically useful, particularly with neoplasm and infectious disease, especially tuberculosis.19,27 One study of 280 hospitalized febrile patients found that bone marrow biopsy was helpful in reaching a diagnosis in nearly 25% of the 130 patients who underwent biopsy.41 Conversely, bone marrow aspiration and culture have a diagnostic yield of only 0% to 2%.3,5,15,22,41
Empiric Therapy and Referral
Empiric trials of antibiotics or steroids rarely establish a diagnosis and are discouraged in the management of patients with FUO, unless there are clinical indications.5,17,19,21,22 Consultation with a subspecialist (e.g., infectious disease specialist, rheumatologist, hematologist/oncologist) is appropriate at any point in the evaluation.
Data Sources: A PubMed search was completed using the key terms fever of unknown origin, FUO, pyrexia of unknown origin, and inflammatory markers. The search included reviews, case series, meta-analyses, and randomized controlled trials. Additional searches included the Cochrane database, Essential Evidence Plus, the Agency for Healthcare Research and Quality evidence reports, and the National Guideline Clearinghouse. Additional references were identified from the articles reviewed. Search dates: November 28, 2011; February 8, 2012; and April 18, 2014.
