Am Fam Physician. 2014;90(5):338-340
Key Points for Practice
Vasomotor symptoms are best managed with systemic HT, although alternatives such as SSRIs, SNRIs, and clonidine have been shown to be effective.
Vaginal symptoms are best treated with systemic or topical HT, but topical methods are preferable as they have fewer adverse effects.
Systemic HT should be given in the lowest dose and for the shortest period possible to decrease the risk of serious adverse events, such as thromboembolic disease and breast cancer.
From the AFP Editors
The median age of menopause in North America is 51 years. There is an array of menopausal symptoms; however, vasomotor and vaginal symptoms are the most closely related to hormonal changes during the transition to menopause. Hot flashes can last one to five minutes; can be characterized by perspiration, flushing, chills, clamminess, anxiety, and on occasion, heart palpitations; and can cause sleep disturbances. Vaginal atrophy results from the menopause-associated hypoestrogenic state and causes anatomic and physiologic changes in the genitourinary tract; symptoms include vaginal or vulvar dryness, discharge, itching, and dyspareunia. The American College of Obstetricians and Gynecologists (ACOG) has provided recommendations for the treatment of vasomotor and vaginal symptoms related to menopause.
Recommendations
BASED ON GOOD OR CONSISTENT EVIDENCE
Systemic estrogen hormone therapy (HT), with or without progestin, is the most effective therapy for menopause-related vasomotor symptoms, with evidence from multiple studies supporting the effectiveness. Oral and transdermal (i.e., patches, gels, or sprays) estrogen, alone or in combination with progestin, can be used, and have been shown to alleviate vasomotor symptoms (Table 1).
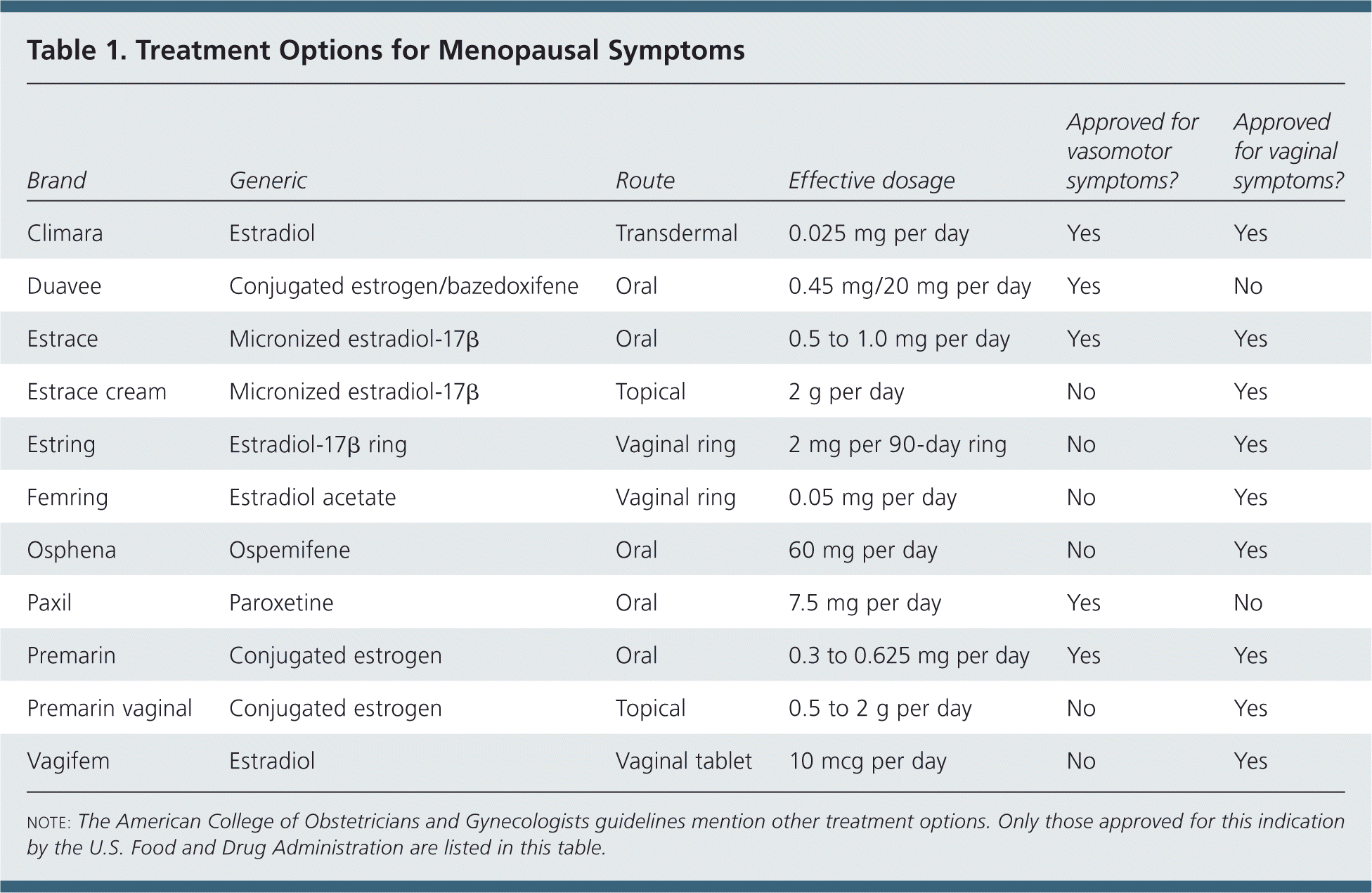
| Brand | Generic | Route | Effective dosage | Approved for vasomotor symptoms? | Approved for vaginal symptoms? |
|---|---|---|---|---|---|
| Climara | Estradiol | Transdermal | 0.025 mg per day | Yes | Yes |
| Duavee | Conjugated estrogen/bazedoxifene | Oral | 0.45 mg/20 mg per day | Yes | No |
| Estrace | Micronized estradiol-17β | Oral | 0.5 to 1.0 mg per day | Yes | Yes |
| Estrace cream | Micronized estradiol-17β | Topical | 2 g per day | No | Yes |
| Estring | Estradiol-17β ring | Vaginal ring | 2 mg per 90-day ring | No | Yes |
| Femring | Estradiol acetate | Vaginal ring | 0.05 mg per day | No | Yes |
| Osphena | Ospemifene | Oral | 60 mg per day | No | Yes |
| Paxil | Paroxetine | Oral | 7.5 mg per day | Yes | No |
| Premarin | Conjugated estrogen | Oral | 0.3 to 0.625 mg per day | Yes | Yes |
| Premarin vaginal | Conjugated estrogen | Topical | 0.5 to 2 g per day | No | Yes |
| Vagifem | Estradiol | Vaginal tablet | 10 mcg per day | No | Yes |
Most women are able to tolerate HT; however, typical doses are associated with adverse effects (e.g., breast tenderness, vaginal bleeding, bloating, headaches). Low- and ultra low-dose estrogen may improve vasomotor symptoms in some women and have better adverse effect profiles compared with the typical doses. There have been limited studies comparing the efficacy of different dosing regimens, but the lower doses do not appear to be as effective as typical dosing. Some women may get significant relief from low-dose therapy, and thus treatment should be individualized. Patients should be treated with the lowest dose and for the shortest period possible to alleviate symptoms.
Thromboembolic disease and breast cancer are risks of combined HT. Most of the studies evaluating the safety of HT were of medications containing conjugated equine estrogen with or without medroxyprogesterone acetate. Other forms of estrogen and progestin may have different risks, such as a lower risk of venous thromboembolism with transdermal estrogen vs. oral estrogen (as indicated by some observational studies). More evidence is needed on the safety and effectiveness of various regimens compared with each other.
Selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), clonidine (Catapres), and gabapentin (Neurontin) are effective nonhormonal medications for treating vasomotor symptoms, although paroxetine (Paxil) is the only nonhormonal medication approved by the U.S. Food and Drug Administration (FDA) for this indication. Adverse effects of SSRIs and SNRIs, which usually go away with time or with a change in dosage, include nausea, dizziness, dry mouth, nervousness, constipation, somnolence, sweating, and sexual dysfunction. Adverse effects of clonidine include dry mouth, insomnia, and drowsiness. Adverse effects of gabapentin include dizziness, somnolence, and peripheral edema.
Estrogen is effective for relieving menopause-related atrophic vaginal symptoms, and all low-dose estrogen is approved by the FDA for this indication. For women with only vaginal symptoms, local therapy is recommended.
Raloxifene (Evista) and tamoxifen, which are FDA-approved for prevention of osteoporosis and breast cancer, are not effective for the treatment of menopause-related vaginal symptoms; evidence does not indicate a significant effect of either for treating vasomotor symptoms. Studies indicate that ospemifene (Osphena), which is FDA-approved for treating moderate to severe dyspareunia in postmenopausal women, improves vaginal atrophy without stimulating the endometrium. Adverse effects of ospemifene include hot flashes, vaginal discharge, muscle spasms, genital discharge, and excessive sweating.
BASED ON LIMITED OR INCONSISTENT EVIDENCE
Data do not support progestin-only medications, testosterone, compounded bioidentical hormones, phytoestrogens, herbal supplements, or lifestyle modifications for the treatment of vasomotor symptoms. Although there are limited data regarding lifestyle modifications, reasonable options include layering clothing, maintaining a lower ambient temperature, drinking cool liquids, and avoiding alcohol and caffeine.
Nonestrogen water- or silicone-based vaginal lubricants and moisturizers may help with menopause-related vaginal symptoms and may be especially helpful in women wanting to avoid HT.
BASED ON CONSENSUS AND EXPERT OPINION
In approximately 50% of women on HT, regardless of age or duration of treatment, discontinuation of HT may cause vasomotor symptom recurrence. Data are insufficient to recommend one discontinuation approach over another (e.g., abrupt, tapering). Regardless of a woman's age, the choice to continue HT should be individualized and should be based on symptoms and the risk-to-benefit ratio.
Guideline source: American College of Obstetricians and Gynecologists
Evidence rating system used? Yes
Literature search described? Yes
Guideline developed by participants without relevant financial ties to industry? Not reported
Published source: Obstetrics & Gynecology, January 2014
Available at: http://journals.lww.com/greenjournal/Abstract/2014/01000/Practice_Bulletin_No__141___Management_of.37.aspx [subscription required]