
A more recent article on enuresis in children is available.
Am Fam Physician. 2014;90(8):560-568
Patient information: See related handout on bed-wetting, written by the authors of this article.
Author disclosure: No relevant financial affiliations.
Enuresis is defined as intermittent urinary incontinence during sleep in a child at least five years of age. Approximately 5% to 10% of all seven-year-olds have enuresis, and an estimated 5 to 7 million children in the United States have enuresis. The pathophysiology of primary nocturnal enuresis involves the inability to awaken from sleep in response to a full bladder, coupled with excessive nighttime urine production or a decreased functional capacity of the bladder. Initial evaluation should include a history, physical examination, and urinalysis. Several conditions, such as constipation, obstructive sleep apnea, diabetes mellitus, diabetes insipidus, chronic kidney disease, and psychiatric disorders, are associated with enuresis. If identified, these conditions should be evaluated and treated. Treatment of primary monosymptomatic enuresis (i.e., the only symptom is nocturnal bed-wetting in a child who has never been dry) begins with counseling the child and parents on effective behavioral modifications. First-line treatments for enuresis include bed alarm therapy and desmopressin. The choice of therapy is based on the child's age and nighttime voiding patterns, and the desires of the child and family. Referral to a pediatric urologist is indicated for children with primary enuresis refractory to standard and combination therapies, and for children with some secondary causes of enuresis, including urinary tract malformations, recurrent urinary tract infections, or neurologic disorders.
Enuresis, or nocturnal enuresis, is defined as urinary incontinence during sleep in a child five years or older.1 It affects 5% to 10% of all seven-year-olds and an estimated 5 to 7 million children in the United States, and it is more common in boys.2–5 There is a spontaneous annual cure rate of 15%, although 2% to 3% of children have symptoms into adulthood.6 This article offers a case-based approach to the evaluation and management of monosymptomatic enuresis in children.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Initial laboratory evaluation for a child with monosymptomatic enuresis should be limited to urinalysis. | C | 3, 9, 23 |
| Daytime symptoms and constipation should be identified and treated before beginning enuresis therapy in children. Secondary causes and contributing factors to enuresis should also be identified and treated appropriately. | C | 3, 9, 24 |
| Bed alarm therapy is effective for the treatment of monosymptomatic enuresis in children. | A | 3, 9, 13, 24, 30 |
| Desmopressin is effective for the treatment of monosymptomatic enuresis in children. | A | 9, 13, 23, 31 |
| Subspecialist referral should be considered in children with primary monosymptomatic enuresis whose symptoms do not respond to alarm therapy or desmopressin; in children who have signs or symptoms of nonmonosymptomatic enuresis or an underlying medical condition; or in children with a suspected comorbid psychiatric disorder. | C | 2, 3, 6, 10, 11, 13, 23, 24 |
Definitions
Enuresis can be subdivided into primary vs. secondary, or monosymptomatic vs. nonmonosymptomatic types. Primary enuresis refers to children who have never achieved six months of continuously dry nights. Secondary enuresis refers to children who previously attained at least six months of nighttime dryness but who have relapsed. Secondary enuresis is more likely to occur with new psychosocial stressors or an underlying medical or behavioral condition.3
In monosymptomatic enuresis, the only symptom is nighttime bed-wetting. Enuresis is often primary and monosymptomatic.7 Nonmonosymptomatic enuresis involves daytime lower urinary tract symptoms (e.g., urgency, frequency, dribbling, incomplete emptying) or daytime incontinence, dysuria, or holding maneuvers (e.g., standing on tiptoes, crossing the legs, pressing the heel or hand into the perineum).6,8 This type of enuresis may suggest an overactive bladder, dysfunctional voiding, or more serious pathology. Children with nonmonosymptomatic enuresis, recurrent urinary tract infections, urinary tract malformations, previous pelvic surgeries, or neurologic disorders should be considered for referral to a subspecialist.9,10
Illustrative Case: Nocturnal Enuresis
The mother of a five-year-old boy is concerned about his nightly bed-wetting. He has never achieved a consistent period of nighttime dryness. He has no daytime wetting, and he denies any dysuria or urgency. What information is needed to make a diagnosis?
EVALUATING ENURESIS
The pathophysiology of primary enuresis involves the inability to awaken from sleep in response to a voiding stimulus (i.e., a full bladder), coupled with excessive nighttime urine production or decreased functional capacity of the bladder.6 When evaluating a child with enuresis, physicians should ask about the frequency, timing, and volume of bed-wetting. They should also ask about daytime lower urinary tract symptoms, because these may not be volunteered by the child or parents who are focused on the bed-wetting. A sense of the child's and parents' concerns about the bed-wetting should be determined, as well as their motivation and desire for treatment. Table 1 provides a list of questions and the clinical responses to the answers.2,6,8,11–13 A bladder diary can be used to assess nighttime voiding patterns, urine output, and daytime drinking habits. Information from the bladder diary can assist in selecting treatment options and assessing response. An example of a bladder diary is available at http://www.hamiltonhealthsciences.ca/documents/Patient%20Education/BladderVoidingDiary-lw.pdf.

| Question | Significance and clinical response |
|---|---|
| General history | |
| What is the child's age? |
|
| Is the child bothered by enuresis? |
|
| Has there been a previous period of 6 months of nighttime dryness? |
|
| Have any therapies been tried previously? |
|
| Enuresis characteristics | |
| What is the frequency of enuresis (days per week and episodes per night)? |
|
| When during the night does enuresis occur? |
|
| Are nighttime absorbent underpants being soaked? |
|
| Does the child have a large first-morning void despite enuresis? |
|
| What are the child's daytime drinking habits, particularly in the afternoon and evening? |
|
| Comorbidities and complicating factors | |
| Does the child have daytime symptoms (e.g., incontinence, urgency, frequency, dribbling, incomplete emptying, straining, weak stream, leakage, holding maneuvers, voiding < 4 or > 7 times per day)? |
|
| Does the child have dysuria? |
|
| Does the child have a history of urinary tract infections? |
|
| Does the child have constipation (< 4 stools per week, hard stools, fecal incontinence)? |
|
| Does the child have behavioral problems? |
|
| Does the child have polydipsia, polyuria, or weight loss? |
|
| Does the child snore or have daytime somnolence? |
|
| Does the child have a history of motor or learning disabilities, or delayed development? |
|
| Are there psychosocial concerns? |
|
Risk factors for enuresis include younger age, male sex, black race, history of urinary tract infection, and a family history of enuresis.14,15 Obesity is also a likely risk factor.16 Low socioeconomic status is not consistently associated with enuresis. Multiple conditions can present with enuresis, either causative or comorbid, and they can influence management and prognosis. Table 2 outlines the most common comorbidities and differential diagnosis.6–8,11,14,15,17–22 Other conditions that may be present include chronic kidney disease, diabetes mellitus, diabetes insipidus, neurogenic bladder, anorexia or other behavioral disorders, sickle cell disease, and hyperthyroidism.11,17
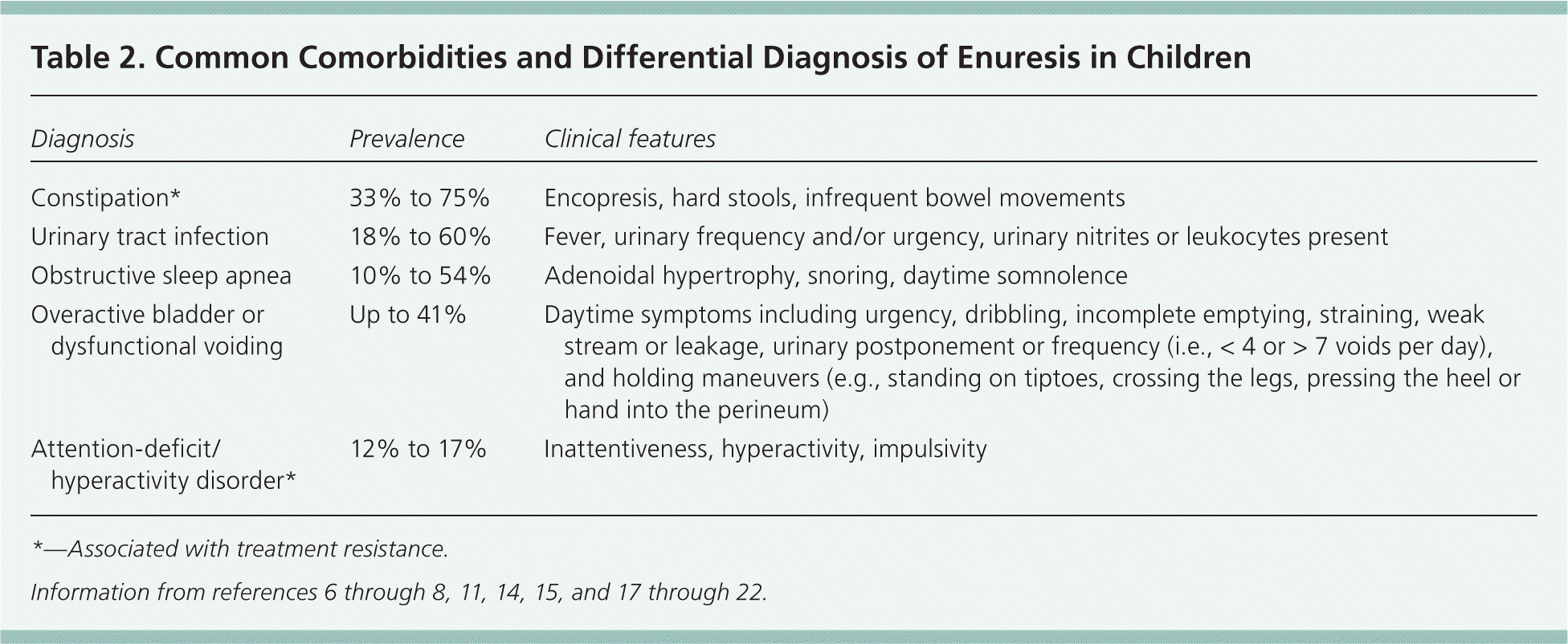
| Diagnosis | Prevalence | Clinical features |
|---|---|---|
| Constipation* | 33% to 75% | Encopresis, hard stools, infrequent bowel movements |
| Urinary tract infection | 18% to 60% | Fever, urinary frequency and/or urgency, urinary nitrites or leukocytes present |
| Obstructive sleep apnea | 10% to 54% | Adenoidal hypertrophy, snoring, daytime somnolence |
| Overactive bladder or dysfunctional voiding | Up to 41% | Daytime symptoms including urgency, dribbling, incomplete emptying, straining, weak stream or leakage, urinary postponement or frequency (i.e., < 4 or > 7 voids per day), and holding maneuvers (e.g., standing on tiptoes, crossing the legs, pressing the heel or hand into the perineum) |
| Attention-deficit/hyperactivity disorder* | 12% to 17% | Inattentiveness, hyperactivity, impulsivity |
The physical examination should focus on identifying causes of secondary (nonmonosymptomatic) enuresis because findings are typically normal in primary enuresis. Genital and rectal examinations can help identify genitourinary malformations or constipation. These examinations may be considered if acceptable to the child and family, and if there are concerns for secondary enuresis. Neurologic examination should include inspection of the lumbosacral spine for signs of occult abnormalities (e.g., dimple, lipoma, hypertrichosis, sacral agenesis), or signs of spinal cord dysfunction, such as tight heel cords, or hammer or claw toes. There should also be an assessment of posturing through stressed gait or mirrored movements to evaluate for central nervous system abnormalities.6 Table 3 summarizes key elements of the physical examination for children with enuresis.2,6,8,11–13

| Finding | Significance and clinical response |
|---|---|
| Height and weight | Growth delay or failure to thrive may suggest an underlying disorder, including chronic kidney disease or diabetes mellitus; further evaluation of these conditions is warranted |
| Head, eyes, ears, nose, and throat | |
| Enlarged adenoids and tonsils | Consider obstructive sleep apnea if snoring and/or daytime somnolence is also present; consider treatment of sleep apnea first |
| Abdomen | |
| Enlarged bladder or kidneys | Suggest anatomic malformations; consider subspecialist referral |
| Hard stool in abdomen | Suggests constipation |
| Genitalia | |
| Physical abnormalities: hypospadias, meatal stenosis, phimosis, labial adhesions | Suggest urinary tract malformations; consider subspecialist referral |
| Signs of sexual abuse | Perianal and perineal excoriations and vulvovaginitis are concerning for sexual abuse and warrant further evaluation |
| Rectum | |
| Evidence of fecal soiling on undergarments | Suggests fecal incontinence and constipation |
| Poor anal sphincter tone | Suggests neurogenic bladder; consider subspecialist referral |
| Dimple, hair tuft, lipoma, or other finding above gluteal cleft | Suggests spinal dysraphism and neurogenic bladder; consider further evaluation and subspecialist referral |
| Nervous system (motor, strength, sensory, reflexes, gait) | |
| Gait abnormality, sensory deficit, abnormal reflexes in lower limbs, tight heel cords, hammer or claw toes | Suggest neurologic disorder; consider further evaluation and subspecialist referral |
DIAGNOSTIC STUDIES
A diagnostic and therapeutic approach to enuresis in children is provided in Figure 1.1–3,6,10,11,13,23,24 For monosymptomatic enuresis, urinalysis is sufficient for an initial laboratory evaluation. Glycosuria or proteinuria suggests diabetes or chronic kidney disease, and warrants further testing with measurement of serum glucose, blood urea nitrogen, and serum creatinine levels. A urine culture should be obtained if bacteria or white blood cells are present.3,9,23
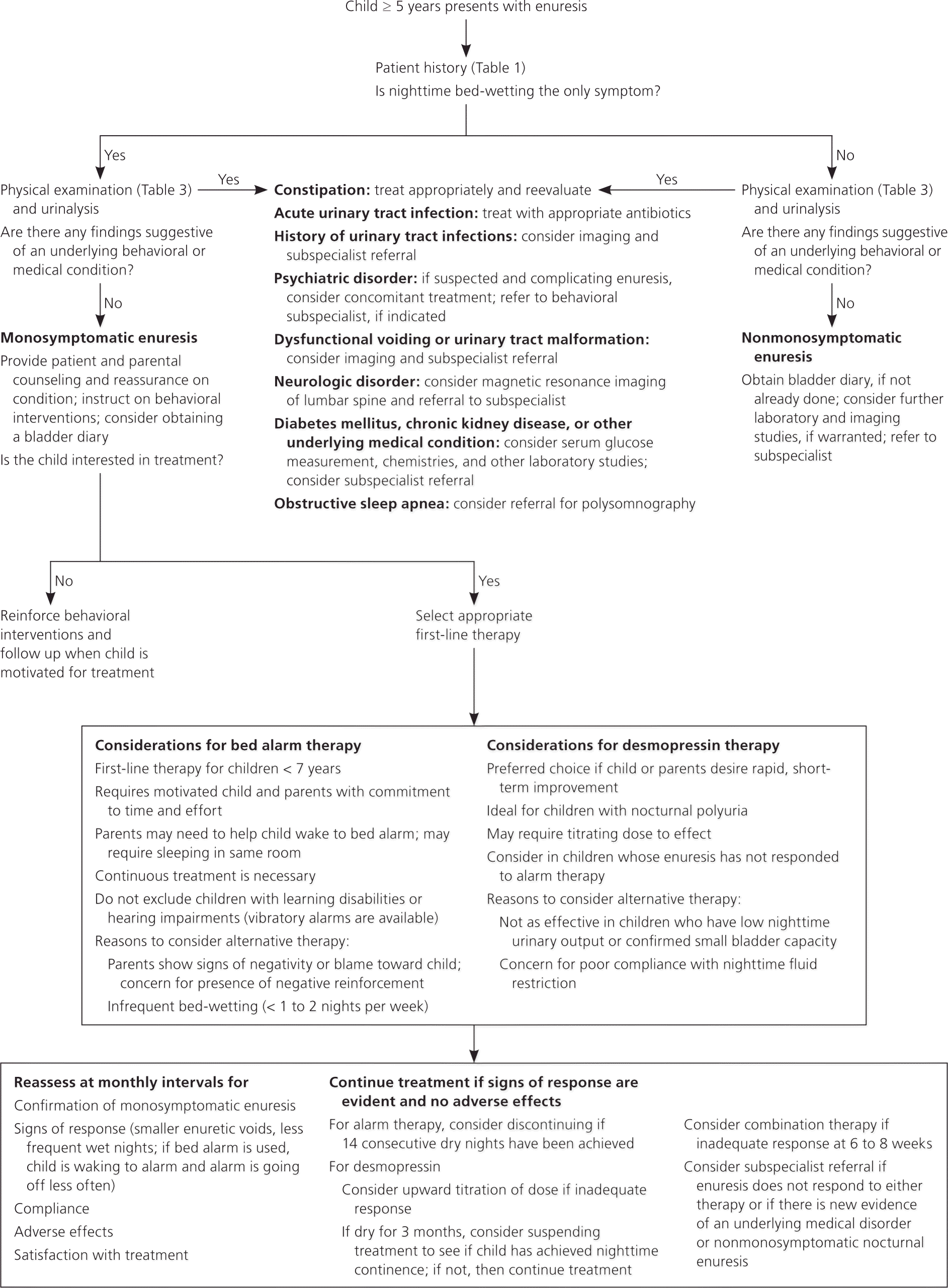
Further diagnostic studies may be indicated for select patients when signs and symptoms suggest nonmonosymptomatic enuresis or an underlying medical condition. Renal ultrasonography can detect kidney disease or anatomic malformations, whereas bladder ultrasonography can evaluate for lower urinary tract malformations, bladder capacity, bladder wall thickness, and postvoid residual urine volume. In addition, bladder ultrasonography is useful in measuring rectal diameter when investigating for constipation. Magnetic resonance imaging is indicated if there is concern for occult spinal dysraphism or other central nervous system disorders. Voiding cystourethrography and urodynamic studies are more invasive diagnostic procedures that are rarely indicated in primary enuresis. They are generally reserved for more complicated cases, and should be performed by subspecialists in enuresis.3,11
Illustrative Case: Primary Monosymptomatic Nocturnal Enuresis
One year ago, primary enuresis was diagnosed in a six-year-old girl. At the time, she was not concerned with her bed-wetting. After discussion with her parents, treatment was deferred until she expressed interest in staying dry at night. Now, her parents report that she has been reluctant to attend sleepovers because of her bed-wetting, and she has been increasingly interested in resolving her bed-wetting. What initial treatments are appropriate for this patient? When should referral be considered?
SUGGESTED MANAGEMENT
Parents should be educated and reassured that primary monosymptomatic enuresis is a common condition that resolves spontaneously in most children. Therefore, parents of younger children may wish to defer treatment other than behavioral interventions, and the physician should support this decision. Counseling and positive reinforcement measures should be discussed.23
Comorbid conditions that can cause or contribute to enuresis should be identified and managed, because the child's response to management may be impaired if other conditions are untreated. Constipation should be treated because enuresis may resolve spontaneously afterward. Obstructive sleep apnea in children is most often caused by enlarged tonsils and adenoids. It is diagnosed by polysomnography, and the primary treatment is adenotonsillectomy. Behavioral or psychiatric disorders should be treated simultaneously with enuresis. Studies suggest that treating attention-deficit/hyperactivity disorder may benefit children with concomitant enuresis, although further research is needed.3,9,24–26
BEHAVIORAL INTERVENTIONS
Simple behavioral interventions can be used as a starting point. Limiting fluid intake in the hours before bed is often encouraged, but has not been systematically studied. Waking the child at night to attempt to urinate; lifting the sleeping child onto the toilet and then waking him or her to urinate; bladder training (e.g., increasing bladder capacity by delaying urination for extended periods, pelvic floor and sphincter control exercises); and instituting reward systems, such as star charts, are all reasonable interventions that appear to be more effective than no treatment, but less effective than bed alarm therapy.27 However, the long-term effectiveness of these methods remains unclear.28
More complex behavioral interventions are more effective. Dry bed training involves a schedule of periodically waking the child to attempt to urinate, “cleanliness training” (having the child change the bedding in the event of an accident), and possible use of a bed alarm. Full-spectrum therapy is a more comprehensive approach that includes bed alarm therapy, cleanliness training, bladder training, and overlearning, which involves having the child drink extra at night after 14 consecutive dry nights are achieved. These interventions are more effective when used in conjunction with a bed alarm, suggesting that much of their benefit is derived from the alarm.29 Negative consequences for wetting should be avoided.30
BED ALARM THERAPY
Bed alarms are currently the most effective treatment available, and are considered first-line therapy for monosymptomatic enuresis.3,9,13,24,30 Many types of alarms are available, but no one type has been shown to be superior. Bed alarms include a moisture sensor, placed on the child's undergarments or as a pad underneath the child, which is connected by wire or wirelessly to an alarm that signals a loud, acoustic stimulus when wetness is detected. The goal is for the child to awaken at the beginning of an enuresis episode, stop urinating temporarily, shut off the alarm, go to the toilet, and finish urinating. Alarms that give an electric shock are generally not acceptable to patients or parents.
The patient's and parents' levels of motivation should be evaluated, because alarms are time intensive and not acceptable for everyone.28 Families who choose to try a bed alarm should commit to a minimum trial of three months. The alarm is usually an out-of-pocket expense, because many health insurance policies do not cover it. After a bed alarm is initiated, follow-up should occur at four weeks to assess for response and compliance, troubleshoot any technical difficulties, and answer any questions. If signs of response (e.g., smaller enuretic voids, fewer wet nights, child waking to the alarm, alarm going off later and less often) are evident within one to three months of use, then therapy should be continued until 14 consecutive dry nights are achieved.13
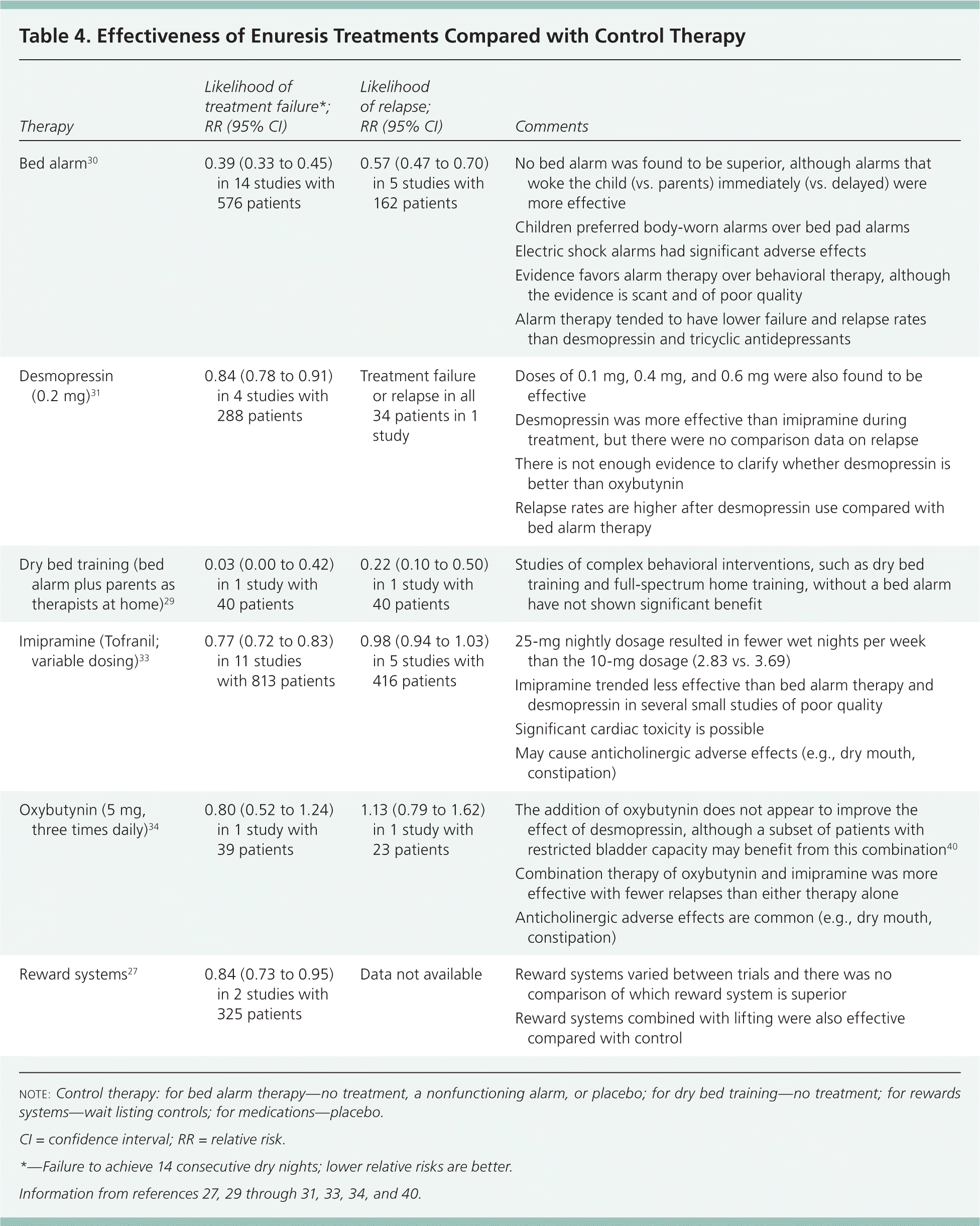
A Cochrane review of 13 trials with 576 patients comparing bed alarm therapy with no treatment or placebo found that the likelihood of treatment failure was much lower with bed alarm therapy than with the control therapy (relative risk = 0.38; 95% confidence interval [CI], 0.33 to 0.45). About one-half of these patients remained dry after discontinuation of alarms compared with almost none in the control group (relapse occurred in 45 of 81 in the treatment group vs. 80 of 81 in the control group). Overlearning is a beneficial addition to alarm training (relative risk = 1.92 for avoiding relapse; 95% CI, 1.27 to 2.92).30
PHARMACOTHERAPY
Pharmacotherapy should be limited to children seven years or older, and can be used if initial nonpharmacologic therapy is unsuccessful or if families prefer medications as initial therapy. Medications are more convenient than behavioral or alarm therapy, but symptoms typically return after discontinuation. The most commonly used medications are desmopressin, tricyclic antidepressants (e.g., imipramine [Tofranil]), and anticholinergics. Desmopressin is an analogue of antidiuretic hormone. It is first-line therapy and the medication of choice, especially in children with nocturnal polyuria and normal bladder capacity.9,13 Desmopressin has a success rate of approximately 70%,23 which is similar to alarm therapy, but the risk of recurrence after discontinuation is higher with desmopressin.
In a Cochrane review of two studies with 119 patients, the relative risk of enuresis recurring after discontinuing desmopressin was 1.42 (95% CI, 1.05 to 1.91) compared with alarm therapy. Patients treated with desmopressin have an average of 1.21 more dry nights per week (95% CI, 0.95 to 1.49) compared with placebo.31 Desmopressin is available as an oral tablet and is generally well tolerated; the intranasal form is no longer recommended in children because of the risks of water intoxication, hyponatremia, and seizures if accidentally overdosed or coupled with excessive nighttime fluid intake.1,3 Follow-up should occur at two to four weeks to assess response and any adverse effects. If no response is achieved at the starting dose of 0.2 mg, it may need to be titrated up to 0.6 mg to achieve dry nights. Desmopressin should be withheld periodically for a short time every three months to assess if nighttime continence has been achieved.2,6 It can also be used on an as-needed basis for events such as sleepovers or camps after an effective dosage is found.
A newer, orally disintegrating desmopressin tablet (DDAVP Melt) is available in Canada and Europe, but not yet in the United States. It is more expensive than the swallowed desmopressin tablet, but may be more effective and preferable to patients. The 0.120-mg disintegrating tablet is the equivalent dose of the 0.2-mg swallowed tablet starting dose.32
Tricyclic antidepressants are considered second-line pharmacotherapy for enuresis. Although they have similar effectiveness to desmopressin, they have a worse adverse effect profile, including the potential for cardiac toxicity in the event of accidental overdose. Most children have recurrence after the discontinuation of tricyclic antidepressants.33
Other medications have been studied to a limited extent. Diclofenac and indomethacin improve enuresis, although neither is as effective as desmopressin.34 Anticholinergics have been used alone or in combination with desmopressin for enuresis not responsive to initial therapies, but are associated with significant adverse effects. They are typically reserved for cases in which low bladder capacity is suspected.23 A wide variety of complementary and alternative medicine therapies have been studied in a limited number of small trials. A Cochrane review determined that there is currently insufficient evidence to recommend any of these therapies for the treatment of enuresis.35
COMBINATION THERAPY
Combination therapy should be considered in the event of treatment failure. Only a few combination therapies have been systematically studied. Bed alarm therapy plus dry bed training is better than either intervention alone.29 A Cochrane review found that the evidence for alarm therapy plus pharmacotherapy is mixed,30 although expert guidelines suggest that if enuresis does not respond or only partially responds to alarm therapy after several months, desmopressin can be combined with alarm therapy.3,13 Almost one-half of patients whose enuresis did not respond to desmopressin became dry when long-acting tolterodine (Detrol LA), 4 mg nightly, was added.36 Limited evidence suggests that pseudoephedrine is effective when combined with ibuprofen or imipramine.37,38 Pelvic floor exercises do not appear to improve full-spectrum therapy.39 Table 4 compares various treatments for enuresis.27,29–31,33,34,40
| Therapy | Likelihood of treatment failure*; RR (95% CI) | Likelihood of relapse; RR (95% CI) | Comments |
|---|---|---|---|
| Bed alarm30 | 0.39 (0.33 to 0.45) in 14 studies with 576 patients | 0.57 (0.47 to 0.70) in 5 studies with 162 patients |
|
| Desmopressin (0.2 mg)31 | 0.84 (0.78 to 0.91) in 4 studies with 288 patients | Treatment failure or relapse in all 34 patients in 1 study |
|
| Dry bed training (bed alarm plus parents as therapists at home)29 | 0.03 (0.00 to 0.42) in 1 study with 40 patients | 0.22 (0.10 to 0.50) in 1 study with 40 patients |
|
| Imipramine (Tofranil; variable dosing)33 | 0.77 (0.72 to 0.83) in 11 studies with 813 patients | 0.98 (0.94 to 1.03) in 5 studies with 416 patients |
|
| Oxybutynin (5 mg, three times daily)34 | 0.80 (0.52 to 1.24) in 1 study with 39 patients | 1.13 (0.79 to 1.62) in 1 study with 23 patients |
|
| Reward systems27 | 0.84 (0.73 to 0.95) in 2 studies with 325 patients | Data not available |
|
Referral to a pediatric urologist is indicated for children with primary monosymptomatic or nonmonosymptomatic enuresis whose symptoms do not improve with standard therapies or who have evidence of urinary tract malformations or recurrent urinary tract infections. Secondary enuresis is typically caused by psychological issues or true urinary tract malformations, and should prompt a thorough workup.2,3,6,11,13,23,24
The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of Defense, the U.S. Army Medical Corps, or the U.S. Army at large.
Data Sources: A PubMed search was conducted using the key term enuresis combined in separate searches with pathophysiology, diagnosis, management, and treatment for enuresis-related topics. The search included clinical reviews, randomized controlled trials, and meta-analyses. Also searched were the Cochrane Database of Systematic Reviews, the National Guideline Clearinghouse database, and Essential Evidence Plus. Relevant publications from the reference sections of cited articles were also reviewed. Search dates: March 9, 2012, and May 16, 2014.
