
A more recent article on community-acquired pneumonia in adults is available.
This is a corrected version of the article that appeared in print.
Am Fam Physician. 2016;94(9):698-706
Related letter: Evidence Poses a Challenge to Imaging Standards in the Diagnosis of Pneumonia
Author disclosure: No relevant financial affiliations.
Community-acquired pneumonia is a leading cause of death. Risk factors include older age and medical comorbidities. Diagnosis is suggested by a history of cough, dyspnea, pleuritic pain, or acute functional or cognitive decline, with abnormal vital signs (e.g., fever, tachycardia) and lung examination findings. Diagnosis should be confirmed by chest radiography or ultrasonography. Validated prediction scores for pneumonia severity can guide the decision between outpatient and inpatient therapy. Using procalcitonin as a biomarker for severe infection may further assist with risk stratification. Most outpatients with community-acquired pneumonia do not require microbiologic testing of sputum or blood and can be treated empirically with a macrolide, doxycycline, or a respiratory fluoroquinolone. Patients requiring hospitalization should be treated with a fluoroquinolone or a combination of beta-lactam plus macrolide antibiotics. Patients with severe infection requiring admission to the intensive care unit require dual antibiotic therapy including a third-generation cephalosporin plus a macrolide alone or in combination with a fluoroquinolone. Treatment options for patients with risk factors for Pseudomonas species include administration of an antipseudomonal antibiotic and an aminoglycoside, plus azithromycin or a fluoroquinolone. Patients with risk factors for methicillin-resistant Staphylococcus aureus should be given vancomycin or linezolid, or ceftaroline in resistant cases. Administration of corticosteroids within 36 hours of hospital admission for patients with severe community-acquired pneumonia decreases the risk of adult respiratory distress syndrome and length of treatment. The 23-valent pneumococcal polysaccharide and 13-valent pneumococcal conjugate vaccinations are both recommended for adults 65 years and older to decrease the risk of invasive pneumococcal disease, including pneumonia.
Together, influenza and pneumonia are the eighth leading cause of mortality among adults in the United States and result in more than 60,000 deaths annually.1–4 Community-acquired pneumonia (CAP) disproportionately affects persons who are very young or very old, with an annual incidence of 9.2 to 33 per 1,000 person-years.1,5 Out of an estimated 878,000 adults 45 years and older who were hospitalized with a primary diagnosis of CAP in 2010, 71% were 65 years or older, and 10% to 20% required admission to the intensive care unit (ICU).1,2,6,7 Pneumococcal pneumonia alone was responsible for 866,000 outpatient visits in 2004.8 In the United States, annual health care costs associated with CAP range from $10.6 to $17 billion and are expected to grow as the proportion of older persons increases.1,2,4 Inpatient care accounts for more than 90% of pneumonia-related health expenditure.2,3,5
WHAT IS NEW ON THIS TOPIC: COMMUNITY-ACQUIRED PNEUMONIA
For patients with severe community-acquired pneumonia, corticosteroids decrease the risk of adult respiratory distress syndrome and modestly reduce intensive care unit and hospital stays, duration of intravenous antibiotic treatment, and time to clinical stability without increasing major adverse events.
Adults 65 years and older should routinely receive the 13-valent pneumococcal conjugate vaccine (PCV13; Prevnar 13) and the 23-valent pneumococcal polysaccharide vaccine (PPSV23; Pneumovax 23), preferably PCV13 first followed by PPSV23 in 12 months.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| In patients with suspected CAP, chest radiography or lung ultrasonography should be performed to confirm the diagnosis. | C | 6, 13, 17, 18 |
| Testing for specific pathogens should be ordered only when it would alter standard empiric therapy, which is rare in outpatients. | C | 6, 19, 22, 25 |
| Use of procalcitonin testing can assist in the management of CAP and reduce antibiotic exposure without compromising patient safety. | C | 27–29 |
| Validated mortality and pneumonia severity assessment tools should be used to determine the appropriate level of care for patients with CAP. | C | 6, 31, 34 |
| Patients with CAP who are admitted to the intensive care unit should be treated with dual antibiotic therapy. | B | 6, 43 |
| For patients with severe CAP, use of corticosteroids within 36 hours improves outcomes. | A | 46–48 |
| Influenza vaccination for all patients and pneumococcal vaccination for patients 65 years and older and other high-risk patients are the mainstays of CAP prevention. | A | 49–51 |
Diagnosis
DIFFERENTIAL DIAGNOSIS
Although Streptococcus pneumoniae remains the most commonly isolated pathogen in CAP, the relative frequency of other pathogens has increased. Clinical suspicion should be driven by comorbidities and other risk factors (Table 1).6,9–11 Commonly used diagnostic methods may identify a pathogen in only 30% to 40% of patients.12

| Risk factor | Related pathogens |
|---|---|
| Alcoholism | Anaerobic oral flora, Klebsiella pneumoniae, Mycobacterium tuberculosis, Streptococcus pneumoniae |
| Aspiration | Anaerobic oral flora |
| Bioterrorism | Bacillus anthracis (anthrax), Francisella tularensis (tularemia), Yersinia pestis (plague) |
| Chronic obstructive pulmonary disease or smoking | Chlamydophila pneumoniae, Haemophilus influenzae, Legionella species,9,10 Moraxella catarrhalis, Pseudomonas aeruginosa or other gram-negative rods, S. pneumoniae |
| Exposure to bat or bird droppings | Histoplasma capsulatum |
| Exposure to farm animals or parturient cats | Coxiella burnetii (Q fever) |
| HIV infection (early) | H. influenzae, M. tuberculosis, S. pneumoniae |
| HIV infection (late) | Aspergillus and Cryptococcus species, H. capsulatum, H. influenzae, Nocardia species, nontuberculous mycobacteria, Pneumocystis jiroveci |
| Hotel or cruise ship travel in past two weeks | Legionella species |
| Influenza active in community | H. influenzae, influenza and other respiratory viruses, S. pneumoniae, Staphylococcus aureus (including MRSA) |
| Injection drug use | Anaerobes, M. tuberculosis, S. aureus (including MRSA), S. pneumoniae |
| Lung abscess | Anaerobic oral flora, M. tuberculosis, nontuberculous mycobacteria, S. aureus (including MRSA) |
| Travel to or residence in Middle East | Middle East respiratory syndrome |
| Travel to or residence in Southeast Asia and East Asia | Avian influenza, severe acute respiratory syndrome |
| Travel to or residence in southeastern and south-central states bordering the Mississippi and Ohio River basins | Blastomyces dermatitidis |
| Travel to or residence in southwestern United States | Coccidioides species, Hantavirus species |
HISTORY AND PHYSICAL EXAMINATION
Most patients with CAP present with a combination of cough, dyspnea, pleuritic pain, fever or chills, and malaise. Risk and severity of CAP, including infection with less common pathogens (e.g., Legionella species), increase with older age, cardiopulmonary disease, poor baseline functional status, low socioeconomic status, and recent weight loss or underweight status.4,9 Although a thorough history is an essential component in the diagnosis of CAP, no individual symptom can adequately predict its presence. Across four studies, the most predictive findings were fever greater than 100°F (37.8°C) (positive likelihood ratio [LR+] is approximately 2.7) and egophony (LR+ = 5.3).13 Clinical prediction rules that combine symptoms and examination findings (Table 214 ) can be helpful in generating a likelihood ratio that can be applied to patients with different prior probabilities of CAP and aid in diagnosis and management.14

| Add points when present | Points | |
| Rhinorrhea | −2 | |
| Sore throat | −1 | |
| Night sweats | 1 | |
| Myalgia | 1 | |
| Sputum all day | 1 | |
| Respiratory rate > 25 breaths per minute | 2 | |
| Temperature ≥ 100°F (37.8°C) | 2 | |
| Total: | _______ | |
| Scoring and likelihood ratios of pneumonia | ||
| Total points | Likelihood ratio | |
| 3 | LR+ = 14.0 | |
| 1 | LR+ = 5.0 | |
| −1 | LR+ = 1.5 | |
| < −1 | LR− = 0.2 | |
Physician judgment has better discriminatory ability to rule out pneumonia (negative likelihood ratio [LR–] = 0.25), but ability to clinically diagnose pneumonia is less certain (LR+ = 2.0).13 Patients with no vital sign abnormality and normal lung examination findings are unlikely to have pneumonia.15 Thus, lung imaging is helpful for patients presenting with possible CAP who have any vital sign or examination abnormalities (given the absence of asthma).16
Guidelines from the Infectious Diseases Society of America advise diagnosing CAP based on suggestive examination findings and characteristic infiltrate on chest radiography with or without microbiologic data.6 Caution should be used when diagnosing CAP in older patients and those who are immunocompromised because they may not exhibit typical symptoms such as a fever greater than 100.4°F (38°C). However, most patients exhibit at least one respiratory symptom; acute functional or cognitive decline; or repeat temperatures of 99°F (37.2°C) or greater.15
IMAGING
Lung imaging with chest radiography has been the standard method of diagnosing pneumonia6 (Figure 1). Lung ultrasonography has the potential to more accurately and efficiently diagnose pneumonia, as well as pleural effusions, pneumothorax, pulmonary embolism, and pulmonary contusions.17 Two recent meta-analyses using chest computed tomography as the comparative standard show that lung ultrasonography has a pooled sensitivity of 0.94 to 0.95 (95% confidence interval [CI], 0.92 to 0.97) and a specificity of 0.90 to 0.96 (95% CI, 0.86 to 0.97), with LR+ = 16.8 (95% CI, 7.7 to 37.0) and LR− = 0.07 (95% CI, 0.05 to 0.10), compared with chest radiography (pooled sensitivity = 0.77 [95% CI, 0.73 to 0.80]; specificity = 0.91 [95% CI, 0.87 to 0.94]) for diagnosis of pneumonia.17,18 Because lung ultrasonography can be performed at the bedside in an average of 13 minutes and lacks ionizing radiation, it is a promising diagnostic option in primary care and emergency department settings.18 Physicians should consider use of chest computed tomography when concern for alternative or concurrent diagnoses remains high (e.g., interstitial lung disease, cavitary lesions, sarcoidosis, malignancy).

DIAGNOSTIC TESTING
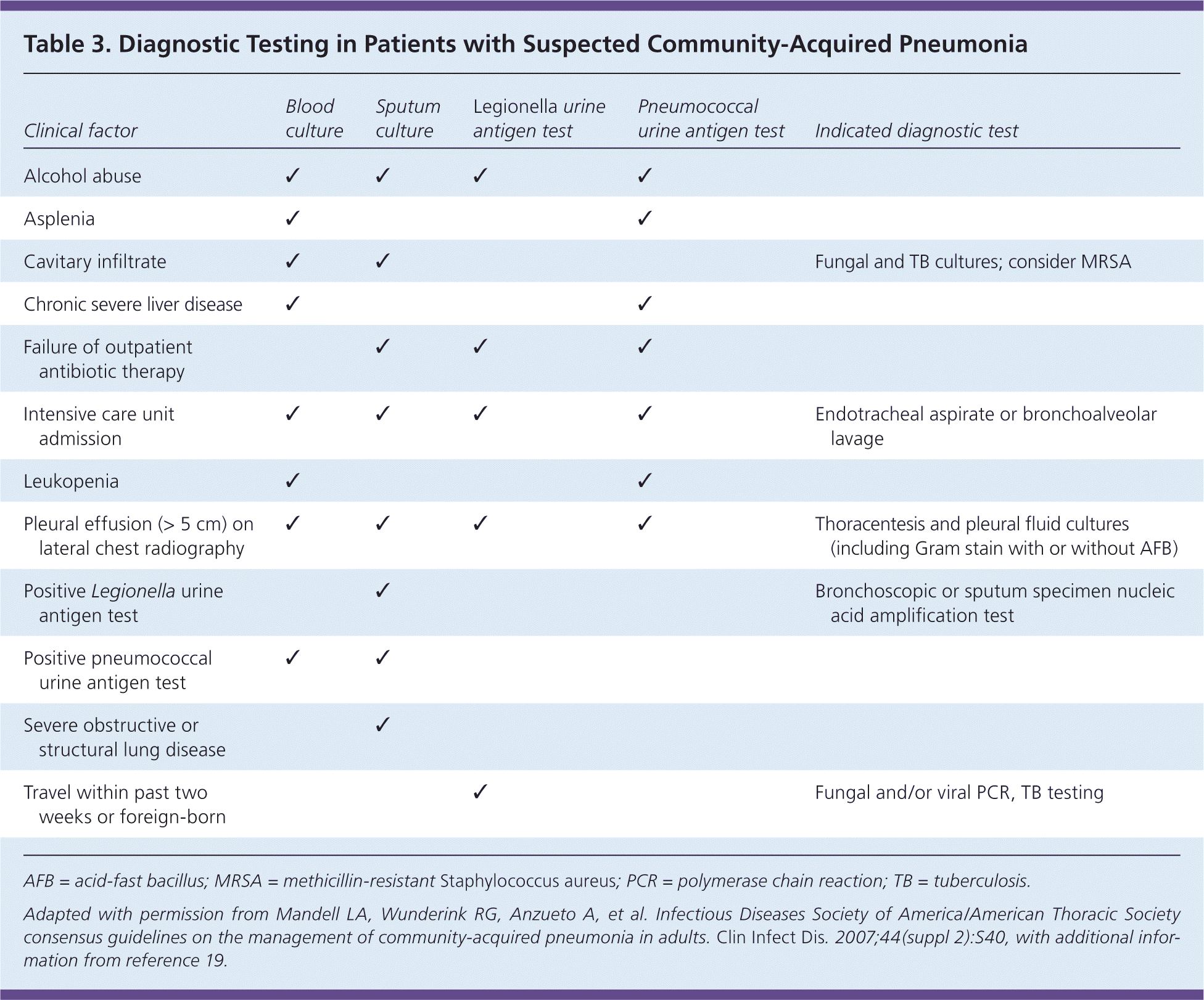
Pulse oximetry should be assessed in all patients with possible CAP. Patients who require supplemental oxygen should receive inpatient treatment. Routine microbiologic testing of outpatients with CAP is unnecessary.6 Testing may be indicated according to patient-specific risk factors (Table 3).6,19 Testing for human immunodeficiency virus infection, tuberculosis, and pneumocystis pneumonia should be considered in those with high-risk sexual exposures or a history of injection drug use. Blood and sputum cultures should be obtained in the presence of specific risk factors or with severe CAP (Table 46,20 ) when culture results can alter antibiotic selection.6 A study of 833 patients with pneumococcal CAP, 47% of whom were bacteremic, showed a marginal but statistically significant increase in the length of stay, time to clinical stability, and in-hospital mortality for bacteremic patients.21 However, an earlier study showed no change in outcomes and demonstrated the potential for false-positive blood cultures to cause prolonged hospitalization and unnecessary vancomycin use.22
| Clinical factor | Blood culture | Sputum culture | Legionella urine antigen test | Pneumococcal urine antigen test | Indicated diagnostic test |
|---|---|---|---|---|---|
| Alcohol abuse | ✔ | ✔ | ✔ | ✔ | |
| Asplenia | ✔ | ✔ | |||
| Cavitary infiltrate | ✔ | ✔ | Fungal and TB cultures; consider MRSA | ||
| Chronic severe liver disease | ✔ | ✔ | |||
| Failure of outpatient antibiotic therapy | ✔ | ✔ | ✔ | ||
| Intensive care unit admission | ✔ | ✔ | ✔ | ✔ | Endotracheal aspirate or bronchoalveolar lavage |
| Leukopenia | ✔ | ✔ | |||
| Pleural effusion (> 5 cm) on lateral chest radiography | ✔ | ✔ | ✔ | ✔ | Thoracentesis and pleural fluid cultures (including Gram stain with or without AFB) |
| Positive Legionella urine antigen test | ✔ | Bronchoscopic or sputum specimen nucleic acid amplification test | |||
| Positive pneumococcal urine antigen test | ✔ | ✔ | |||
| Severe obstructive or structural lung disease | ✔ | ||||
| Travel within past two weeks or foreign-born | ✔ | Fungal and/or viral PCR, TB testing |

| Minor criteria | |
| Confusion or disorientation | |
| Hypotension requiring aggressive fluid resuscitation | |
| Multilobar infiltrates | |
| PaO2/FiO2 ratio ≤ 250 | |
| Respiratory rate ≥ 30 breaths per minute | |
| Urea nitrogen level ≥ 20 mg per dL (7.14 mmol per L) | |
| Minor criteria that have less prognostic value20 | |
| Hypothermia (core temperature < 96.8°F [36°C]) | |
| Leukopenia (white blood cell count < 4,000 per mm3 [4.0 × 109 per L]) | |
| Thrombocytopenia (platelet count < 100 × 103 per mm3 [100 × 109 per L]) | |
| Major criteria | |
| Invasive mechanical ventilation | |
| Septic shock with need for vasopressors | |
Urine antigen testing may be useful in select patients, especially if adequate sputum cannot be obtained or antibiotics were given, because antigen can be detected starting on the first day of illness and for several weeks thereafter.6,23 Pneumococcal urine antigen testing has a sensitivity of 60% to 80% and specificity greater than 90%, whereas Legionella testing has a sensitivity of 70% to 90% and specificity of 99% for serogroup 1, which accounts for 80% to 95% of Legionella-associated CAP.6 Testing is most useful in critically ill patients and in the scenarios listed in Table 3.6,19
The overall rate of pathogen detection among patients with CAP is 30% to 40%.12 Recent developments allow use of rapid multiorganism polymerase chain reaction–based testing for pathogen-directed treatment, with bacterial and viral detection rates as high as 86%.9,19 Such detection can potentially result in earlier de-escalation of therapy and decreased reliance on broad-spectrum antimicrobials.11,24 Additionally, because viral etiologies account for about 25% of all CAP cases, utilization of rapid viral respiratory panels can be useful to tailor treatment and limit antibiotic exposure.6,24–26
To improve patient outcomes and reduce unnecessary antibiotic exposure, procalcitonin is being studied as a potential biomarker of severe CAP. With a cost of $25 to $30 per test, the value of procalcitonin testing is dependent on laboratory capability, with a cutoff value of less than 0.1 ng per mL having superior prognostic accuracy for short-term mortality (relative risk = 4.18; 95% CI, 3.19 to 5.48), bacteremia, and disease severity.27,28 A 2012 Cochrane meta-analysis found the use of procalcitonin testing significantly decreased median antibiotic exposure from eight to four days and lowered the risk of treatment failure (19.1% vs. 21.9%) with no impact on mortality or length of hospitalization, regardless of clinical setting.29
A systematic review of 14 randomized controlled trials examining use of procalcitonin testing in European settings found a reduction in antibiotic prescribing in low-acuity settings and shorter duration of therapy in higher-acuity settings (i.e., emergency department and ICU) when results of high-sensitivity (detection limit 0.1 ng per mL) procalcitonin assays were used in clinical decision making.30 Further research is needed to evaluate the impact of procalcitonin testing among patients with acute respiratory illnesses in terms of patient-oriented outcomes, antibiotic exposure, and cost-effectiveness in U.S. populations.
Management
INPATIENT VS. OUTPATIENT CARE
The costs of hospitalization and potential for nosocomial infections and thromboembolic events necessitate careful consideration of risk factors for severe CAP when considering appropriate level of care (Table 4).6,20 Decision support tools such as the Pneumonia Severity Index (https://www.aafp.org/afp/2006/0201/p442.html#afp20060201p442-t3) assist in predicting the risk of 30-day mortality and severe CAP requiring ICU admission. Table 5 summarizes the more widely adopted CURB-65 and CRB-65 prediction scores,6,31 which have greater ease of use, but weaker predictive power for 30-day mortality.24,31,32 Among low-risk patients (Pneumonia Severity Index class less than 3 or CURB-65 score less than 2), the LR− for 30-day mortality using the index is 0.08, and 0.21 using CURB-65.31 Sensitivity for predicting ICU admission was reported as 74% vs. 39% to 50% for Pneumonia Severity Index vs. CURB-65, respectively.24,32

| Prognostic variables (assign 1 point for each variable) | ||||||
| Confusion (new onset) | ||||||
| Urea nitrogen level > 20 mg per dL (7.14 mmol per L)* | ||||||
| Respiratory rate ≥ 30 breaths per minute | ||||||
| Blood pressure (systolic < 90 mm Hg or diastolic ≤ 60 mm Hg) | ||||||
| Age ≥ 65 years | ||||||
| 30-day mortality for CURB-65 (CRB-65) | ||||||
| Score | Sensitivity | Specificity | LR+ | LR– | ||
| ≥ 1 point | 98.6% (94.4%) | 26.5% (38.3%) | 1.2 (1.3) | 0.10 (0.15) | ||
| ≥ 2 points | 89.1% (72.7%) | 52.2% (70.8%) | 1.7 (2.4) | 0.21 (0.39) | ||
| ≥ 3 points | 62.0% (29.1%) | 80.8% (90.9%) | 3.1 (4.4) | 0.46 (0.72) | ||
| ≥ 4 points | 29.0% | 95.3% | 5.4 | 0.73 | ||
| 30-day observed mortality by score | ||||||
| CURB-65† | CRB-65‡ | |||||
| Score | Observed mortality | Score | Observed mortality | |||
| 0 to 1 | 2.0% | 0 | 2.3% | |||
| 2 | 8.3% | 1 to 2 | 13.3% | |||
| 3 to 5 | 22.3% | 3 to 4 | 34.4% | |||
Although CURB-65 has a higher false-negative rate (i.e., incorrectly ruling out severe CAP) compared with other instruments, its relatively high specificity allows physicians to more accurately advance treatment for patients with a score of 3 or greater while taking further measures to determine risk in patients who score low.24,32,33 CRB-65 is more practical for use in the primary care setting without a significant compromise in prognostic value.6,31 Patients with a score of 0 or 1 can be managed safely as outpatients.6
SMART-COP (https://www.aafp.org/afp/2011/0601/p1299.html#afp20110601p1299-t6), a more recently developed predictive tool, was designed to identify patients with CAP who are most likely to need mechanical ventilation or vasopressor support (sensitivity = 92%).34 Other risk factors predictive of severe CAP include male sex and the presence of congestive heart failure or chronic obstructive pulmonary disease.35
ANTIBIOTIC THERAPY

| Patient group | Recommended initial therapy | |
|---|---|---|
| Previously healthy outpatients with no antibiotic use in past three months | Macrolide (azithromycin [Zithromax]) or doxycycline | |
| Outpatients with comorbidities* or antibiotic use in past three months† | Preferred36,37: Respiratory fluoroquinolone (levofloxacin [Levaquin], gemifloxacin [Factive], or moxifloxacin [Avelox]) | |
| Alternative: Beta-lactam antibiotic (high-dose amoxicillin, amoxicillin/clavulanate [Augmentin], cefuroxime [Ceftin], or cefpodoxime) plus a macrolide‡ | ||
| Inpatients, non-ICU§ | Preferred36,37: Respiratory fluoroquinolone | |
| Alternative: Beta-lactam antibiotic plus a macrolide | ||
| Inpatients, ICU | Beta-lactam antibiotic (ceftriaxone, cefotaxime [Claforan], or ampicillin/sulbactam [Unasyn]), plus a macrolide alone or a macrolide and a respiratory fluoroquinolone||¶ | |
| Special considerations | ||
| Risk factors for Pseudomonas species | Beta-lactam antibiotic (piperacillin/tazobactam [Zosyn], cefepime, imipenem/cilastatin [Primaxin], meropenem [Merrem IV], or doripenem [Doribax]), plus either ciprofloxacin or levofloxacin | |
| or | ||
| The above beta-lactam antibiotic plus an aminoglycoside and azithromycin | ||
| or | ||
| The above beta-lactam antibiotic plus an aminoglycoside and an antipneumococcal respiratory fluoroquinolone | ||
| Risk factors for methicillin-resistant Staphylococcus aureus | Vancomycin, linezolid (Zyvox), or ceftaroline (Teflaro)38 | |
| Influenza virus | Oseltamivir (Tamiflu) or zanamivir (Relenza) | |
Outpatient. Evidence is lacking on the optimum antibiotic regimen for treatment of CAP in the outpatient setting.41 In the absence of significant comorbidities, macrolides (e.g., azithromycin [Zithromax]) should be used for outpatient therapy of adult CAP, with doxycycline as an alternative, because S. pneumoniae and atypical pathogens account for most cases of CAP, especially in North America.6,16 Exposure to antibiotics in the preceding three months or presence of comorbid illness necessitates the preferential use of fluoroquinolones followed by a beta-lactam antibiotic (e.g., high-dose amoxicillin, amoxicillin-clavulanate [Augmentin], cefuroxime [Ceftin], cefpodoxime) combined with a macrolide as an alternative. Evidence from randomized controlled trials shows a modest reduction in treatment failure, adverse events, and treatment discontinuation with fluoroquinolones compared with combination beta-lactam and macrolide antibiotics.36,37
Facility-specific antibiograms should be used whenever available to account for variable resistance patterns. Given the risk of pneumococcal resistance, an antibiotic from a different class should be used when recent antibiotic exposure is known. A five-day treatment course for low-severity CAP has been shown to be sufficient in clinical trials, whereas a seven- to 10-day course should be provided in patients with moderate- and high-severity CAP (based on CURB-65 and CRB-65 scores).42
Inpatient, No ICU. Hospitalized patients who are not admitted to the ICU should receive a respiratory fluoroquinolone or a beta-lactam antibiotic and a macrolide. Observational data suggest lower short-term mortality when antibiotic therapy is administered within four to eight hours of hospital arrival in patients with moderate or severe pneumonia.36 Table 7 provides recommended criteria for transitioning patients from intravenous to oral antibiotics.36

| Able to ingest oral agents |
| Heart rate < 100 beats per minute and systolic blood pressure > 90 mm Hg |
| Oxygen saturation > 90%, arterial oxygen partial pressure > 60 mm Hg on room air or with low-flow supplemental oxygen via nasal cannula, or return to baseline oxgen level for patients receiving long-term oxygen therapy |
| Respiratory rate < 25 breaths per minute |
| Return to baseline cognitive status |
| Temperature < 100.9°F (38.3°C) |
Although oral doxycycline is not included in existing guidelines, observational studies show that it has similar effectiveness as levofloxacin (Levaquin) alone or in combination with ceftriaxone in the inpatient, non-ICU setting, and is associated with fewer Clostridium difficile infections.39,40,43,44 Trials of a new cephalosporin (ceftaroline [Teflaro]) show promising results, although treatment should be reserved for patients with highly resistant gram-positive organisms.38
Inpatient, ICU. Patients with severe CAP who are admitted to the ICU—about 10% of patients hospitalized with CAP—should receive dual antibiotic therapy, which has been shown to improve survival6,7,43 (Table 66,36–40 ). Anaerobic coverage should be considered if risk factors are present, including aspiration, stroke with bulbar symptoms, alcoholism, and loss of consciousness. Such agents include carbapenems, clindamycin, metronidazole (Flagyl), amoxicillin/clavulanate, and piperacillin/tazobactam (Zosyn).7 Coverage for methicillin-resistant Staphylococcus aureus and Pseudomonas species should be administered depending on risk factors (Table 16,9–11 and Table 36,19 ). Physicians must be aware that up to one in four cases of CAP has a viral etiology, which may account for poor response to antibiotics or atypical features.6,26 Prompt recognition and antiviral treatment, especially during influenza season, improve outcomes and reduce mortality.45
ADJUNCTIVE THERAPIES
Meta-analyses of randomized trials of corticosteroids for CAP demonstrate a decreased risk of adult respiratory distress syndrome (relative risk = 0.21; 95% CI, 0.08 to 0.59) and modest reductions in lengths of ICU and hospital stays, duration of intravenous antibiotic treatment, and time to clinical stability without a corresponding increase in major adverse events.46 Additional observations include reduced need for mechanical ventilation (number needed to treat = 20) and 3% reduction in mortality with a typical regimen consisting of methylprednisolone 0.5 mg per kg every 12 hours for five to seven days, administered within 36 hours of admission.47,48
Prevention
The 2015 Advisory Committee on Immunization Practices recommends that adults 65 years and older receive both the 13-valent pneumococcal conjugate vaccine (PCV13; Prevnar 13) and 23-valent pneumococcal polysaccharide vaccine (PPSV23; Pneumovax 23), preferably with PCV13 administered first, followed by PPSV23 in 12 months.49 If PPSV23 was already given after age 65, PCV13 should be offered after one year. When PPSV23 is administered before age 65, PCV13 should be offered if at least one year has elapsed, followed by another dose of PPSV23 after at least 12 months and if at least five years have elapsed following the previous PPSV23 dose.
In the presence of immunocompromising conditions (e.g., functional or anatomic asplenia, cochlear implants, cerebrospinal fluid leak), a minimum of eight weeks between doses of PCV13 before PPSV23 is recommended.49 In adults 19 to 64 years of age, a dose of PPSV23 should be given in the presence of diabetes mellitus, chronic lung or heart disease, tobacco use, alcoholism, chronic liver disease or cirrhosis, cerebrospinal fluid leaks, or cochlear implant.50 Additionally, either PPSV23 or PCV13 can be safely co-administered with the influenza vaccine.26 Evidence supports the influenza vaccine in preventing pneumonia, hospitalization, and death, particularly among older patients.51
Data Sources: A PubMed search was completed in Clinical Queries using the key term community-acquired pneumonia. The search included meta-analyses, randomized controlled trials, clinical trials, practice guidelines, and reviews. The limits included English language, humans, and all adults 18 years and older. We also searched Best Evidence Review, Cochrane Database of Systematic Reviews, National Guideline Clearinghouse, and the U.S. Preventive Services Task Force. Search dates: October 2015 through February 2016.
