Am Fam Physician. 2017;96(8):507-514
Patient information: See related handout on bladder cancer.
Author disclosure: No relevant financial affiliations.
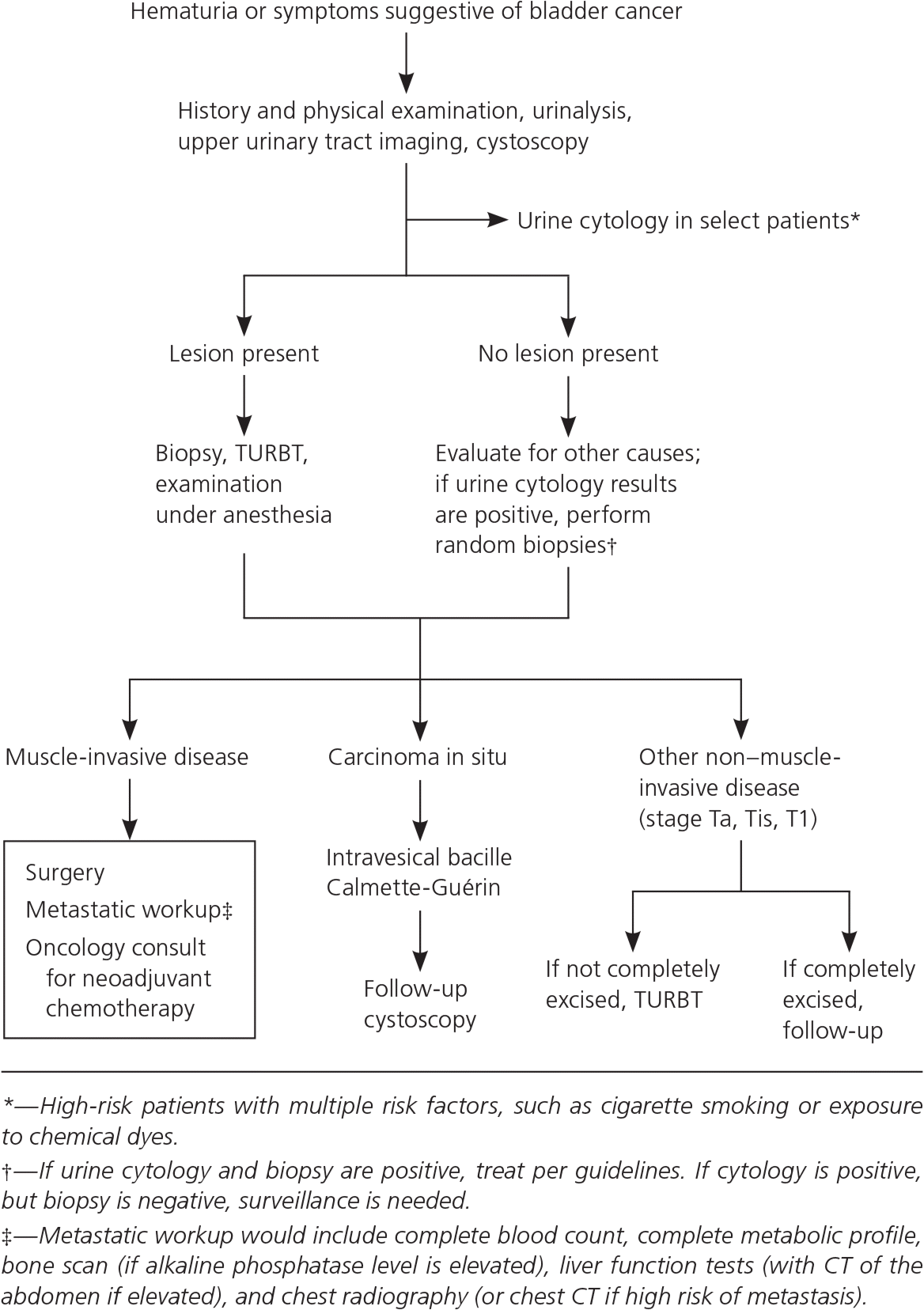
Bladder cancer is the sixth most prevalent malignancy in the United States and causes more than 16,000 deaths annually. The most common clinical presentation is asymptomatic hematuria, which should prompt evaluation with cystoscopy, renal function testing, and upper urinary tract imaging in adults 35 years and older and in those with irritative voiding symptoms, risk factors for bladder cancer, or gross hematuria at any age. Transurethral resection of the bladder tumor allows for definitive diagnosis, staging, and primary treatment. Non–muscle-invasive disease is treated with transurethral resection, most often followed by intravesical bacille Calmette-Guérin or intravesical chemotherapy. Bladder cancer that invades the muscle layer is typically treated with radical cystectomy and neoadjuvant chemotherapy because of higher rates of progression and recurrence. No major organization recommends screening asymptomatic adults for bladder cancer, and the U.S. Preventive Services Task Force concluded that current evidence is insufficient to assess the balance of benefits and harms of screening.
Bladder cancer comprises 5% of new cancer diagnoses in the United States and is the sixth most prevalent malignancy.1 About 90% of affected patients are older than 55 years, with a mean age at diagnosis of 73 years.1 Men are three to four times more likely than women to develop the disease, with a lifetime risk of one in 26 for men and one in 88 for women.1 Bladder cancer affects whites about two times more often than blacks or Hispanics, but it is more likely to be diagnosed at an advanced stage in black patients.1 As the incidence of the disease has decreased, bladder cancer–related mortality has decreased for women but remains unchanged for men.1 An estimated 16,400 deaths were caused by bladder cancer in 2016.1
| Clinical recommendation | Evidence rating | References | Comments |
|---|---|---|---|
| Urine cytology and testing for urine tumor markers should not be performed routinely for evaluation of bladder cancer. | C | 21, 29 | Urine cytology has > 90% sensitivity for detection of high-grade tumors and carcinoma in situ; it can be of benefit in patients with high pretest probability of disease. |
| Cystoscopy should be performed in all patients with gross hematuria, all patients 35 years and older who have microscopic hematuria, and all patients with symptomatic microscopic hematuria of unclear etiology, regardless of age. | C | 21 | — |
| Initial evaluation for bladder cancer should include imaging of the upper urinary tract. | C | 27, 30 | Computed tomography urography is preferred. |
| Non–muscle-invasive bladder cancer should be treated with transurethral resection of the tumor, with or without intravesical instillation of immunotherapy or chemotherapy. | C | 20, 27, 32 | — |
| Cisplatin-based neoadjuvant chemotherapy should be considered in addition to radical cystectomy with extended lymphadenectomy for additional survival benefit in patients with muscle-invasive bladder cancer. | A | 29 | There is an absolute five-year survival benefit of 5% to 8%. |
| The U.S. Preventive Services Task Force concludes the current evidence is insufficient to assess the balance of benefits and harms of screening for bladder cancer in asymptomatic adults. | C | 33 | The American Academy of Family Physicians concurs. |

| Recommendation | Sponsoring organization |
|---|---|
| Do not diagnose microhematuria solely on the results of urine dipstick testing (macroscopic analysis). | American Urological Association |
Risk Factors
Established risk factors for bladder cancer include male sex, older age, white race, occupational exposure to certain chemicals, pelvic radiation, use of medications such as cyclophosphamide, chronic bladder infection/irritation, personal or family history of bladder cancer, and cigarette smoking2 (Table 12–16 ). Studies have also suggested additional associations including diabetes mellitus, obesity, and human papillomavirus.8,14,15 Use of pioglitazone (Actos) for more than one year is independently associated with a slightly increased risk of bladder cancer.13 Consuming large amounts of processed red meat may also slightly increase risk.4

| Behavioral |
| Cigarette smoking2,3 |
| Consumption of processed red meat*4 |
| Chemical exposure |
| Arsenic-contaminated water2 |
| Chronic infection/irritation |
| Chronic kidney and bladder stones5 |
| Chronic urinary tract infections2 |
| Indwelling catheter (long-term)2,6 |
| Schistosomiasis2,7 |
| Genetics |
| Personal or family history of bladder cancer, especially at an early age2,8 |
| Iatrogenic |
| Aristolochia herbal preparations2 |
| Computed tomography in childhood or adolescence9 |
| Cyclophosphamide2,10,11 |
| Pelvic radiation therapy2,6,11 |
| Phenacetin (no longer available in the United States)12 |
| Pioglitazone (Actos) use for more than one year*13 |
| Medical conditions |
| Diabetes mellitus*14 |
| Human papillomavirus infection*15 |
| Obesity*8 |
| Renal transplant recipient*10 |
| Occupational exposures |
| Exposure to aromatic amines (beta-naphthylamine) used in the manufacturing of chemical dyes and pharmaceuticals, and in gas treatment plants 2,3,7,8 |
| Farming in Africa and the Middle East (increased risk of schistosomiasis)7 |
| Manufacturing (cable, paint, petroleum, tire and rubber, textile); service industry (hairdressers, painters, truck drivers)3,7,16 |
Pathology
Urothelial (transitional cell) carcinoma is the most common type of bladder cancer, accounting for approximately 90% of cases 17 (eTable A). Nonurothelial bladder cancers include squamous cell carcinoma, adenocarcinoma, small cell carcinoma, and mixed histology tumors, with squamous cell and adenocarcinomas making up the majority of nonurothelial tumors.18 Although squamous cell carcinoma represents only a small fraction of bladder cancer cases in the developed world, it is the most common type in places where schistosomiasis is endemic, accounting for up to 81% of cases in these areas.19

| Epithelial neoplasms (99%) | |||
| Urothelial (transitional cell) (90%) | |||
| Invasive urothelial carcinoma: lamina propria or muscularis propria invasion | |||
| Papillary urothelial carcinoma: low malignant potential, low-grade, or high-grade | |||
| Papilloma: flat or papillary | |||
| Nonurothelial (9%) | |||
| Adenocarcinoma | |||
| Rare neoplasms | |||
| Small cell carcinoma | |||
| Squamous cell carcinoma | |||
| Nonepithelial (mesenchymal) neoplasms (1%) | |||
| Benign | |||
| Hemangioma | |||
| Leiomyoma | |||
| Lipoma | |||
| Neurofibroma | |||
| Paraganglioma | |||
| Malignant | |||
| Angiosarcoma | |||
| Leiomyosarcoma | |||
| Malignant fibrous histiocytoma | |||
| Osteosarcoma | |||
| Rhabdomyosarcoma | |||
Clinical Presentation
Painless hematuria is the most common presenting sign of bladder cancer 20,21 (Table 222 ). Approximately 1.3% of patients with asymptomatic microscopic hematuria (three or more red blood cells per high-power field in a properly collected specimen in the absence of an obvious benign cause 20,21) will have bladder cancer, with estimates ranging from 0.4% to 6.5%.20 Gross hematuria is associated with a higher rate of malignancy (estimated at 20%).23,24 Irritative voiding symptoms are often present (e.g., urinary frequency, urgency, nocturia, dysuria).20 Obstructive symptoms, such as reduced or intermittent urine stream, straining, or feeling of incomplete voiding, may be present if the tumor is near the bladder neck or urethra.20

| Hematuria (gross or microscopic) |
| Irritative symptoms (e.g., dysuria, frequency, nocturia, urge incontinence, urgency) |
| Obstructive symptoms (e.g., decreased force of stream, feeling of incomplete voiding, intermittent stream, straining) |
| Signs and symptoms of metastases or advanced disease (e.g., abdominal, bone, flank, or pelvic pain; anorexia, cachexia, or pallor; lower-extremity edema; renal failure; respiratory symptoms; suprapubic palpable mass) |
Patients with advanced disease may present with symptoms related to metastatic involvement.22,25 The most common sites for metastasis include the lymph nodes, bone, lung, liver, and peritoneum.26 Physical examination findings are often unremarkable in patients with bladder cancer unless there is advanced or metastatic disease, which may demonstrate a palpable renal or bladder mass.20
Diagnosis

LABORATORY TESTS
To evaluate for renal impairment, serum blood urea nitrogen and creatinine levels should be obtained for all patients in whom bladder cancer is suspected.21 If metastatic disease is suspected, a complete blood count and complete metabolic panel, including alkaline phosphatase level and assessment of liver function, are appropriate.21
CYTOLOGY AND URINARY TUMOR MARKERS
Urine cytology is not recommended as part of the routine evaluation for asymptomatic microscopic hematuria21 because renal calculi and urinary tract infections can lead to false-positive results.29 It has high sensitivity (greater than 90%) for high-grade urothelial tumors and carcinoma in situ (stage Tis), although negative findings do not rule out malignancy.29 Urine cytology is an important adjunct in the evaluation of patients at high risk of urothelial tumors because of its positive predictive value in these patients.21 Urine cytology is also useful for surveillance in patients with known urothelial carcinoma.21 Routine testing for urinary tumor markers is not recommended because of lack of clinical reliability.21
CYSTOSCOPY AND TURBT
Cystoscopy should be performed in all patients with gross hematuria and in those 35 years and older with microscopic hematuria, and it can be considered for younger patients with microscopic hematuria.21 Patients with hematuria and risk factors for bladder cancer (e.g., tobacco use, irritative voiding symptoms, chemical exposures) should be evaluated with cystoscopy regardless of age.21
Patients with abnormal findings on bladder wash cytology or tissue pathology should undergo transurethral resection of the bladder tumor (TURBT). This procedure provides essential histopathologic information necessary for definitive diagnosis, staging, and grading, and allows for removal of visible tumor and sampling of surrounding muscle to assess depth.20
UPPER URINARY TRACT IMAGING
Multiphasic CT urography with and without intravenous contrast media and excretory imaging should be included in the initial workup for bladder cancer.27 It has the highest sensitivity (95%) and specificity (92%) of all upper urinary tract imaging modalities, and it can evaluate the urothelium of the upper tracts as well as detect renal masses.30 Magnetic resonance urography and ultrasonography are alternative imaging options for patients with contraindications to CT urography, such as pregnancy, contrast allergy, or renal insufficiency.21 Renal ultrasonography may be considered in addition to CT urography for patients who have suspected renal parenchymal disease.30 Renal ultrasonography is not recommended on its own for evaluation of hematuria without a known cause, because its low sensitivity (50%) may miss urothelial lesions, small renal masses, or renal calculi, and it often leads to further testing and additional costs.28 If metastatic disease is suspected, chest radiography and imaging of the abdomen and pelvis with CT or magnetic resonance imaging should be obtained.31
Screening
No major organizations recommend screening for bladder cancer in asymptomatic adults.5,17,20,27,32 In 2011, the U.S. Preventive Services Task Force concluded that there was insufficient evidence to assess the balance of benefits and harms of screening for bladder cancer in asymptomatic adults,33 a statement endorsed by the American Academy of Family Physicians.34 The low positive predictive value of available screening tests and lack of high-quality evidence to suggest that early treatment of bladder cancer improves long-term outcomes preclude a recommendation in favor of screening.
Initial Treatment
Management of bladder cancer depends on the pathologic extent of disease at the time that TURBT is performed and on subsequent staging according to the tumor-node-metastasis classification system (eTable B and Figure 235 ). Non–muscle-invasive disease is most often treated with TURBT followed by single-dose intravesical immunotherapy with bacille Calmette-Guérin (BCG) or intravesical chemotherapy in tumors with greater risk of progression or recurrence. CT or magnetic resonance imaging is often used to evaluate invasion beyond the bladder. Imaging should be done before TURBT is performed because of the possibility of a post-TURBT perivesicular reaction, which can make interpretation difficult.17 Because of the increased risk of progression and recurrence, bladder tumors that invade the muscle are typically treated by radical cystectomy with extended lymphadenectomy, preceded by cisplatin-based neoadjuvant chemotherapy.17,36 Bladder preservation is an option in certain patients, usually followed by chemotherapy and radiation.17

| Primary tumor (T) | |||
| TX: Primary tumor cannot be assessed | |||
| T0: No evidence of primary tumor | |||
| Ta: Noninvasive papillary carcinoma | |||
| Tis: Carcinoma in situ (“flat tumor”) | |||
| T1: Tumor invades subepithelial connective tissue | |||
| T2: Tumor invades muscularis propria (2a superficial inner half; 2b deep outer half) | |||
| T3: Tumor invades perivesical tissue (3a microscopically; 3b macroscopically) | |||
| T4: Tumor invades any of the following: prostate, uterus, vagina, pelvic wall, abdominal wall | |||
| T4a: Tumor invades prostate, uterus, vagina | |||
| T4b: Tumor invades pelvic wall, abdominal wall | |||
| Regional lymph nodes (N)* | |||
| NX: Regional lymph nodes cannot be assessed | |||
| N0: No lymph node metastasis | |||
| N1: Single regional lymph node metastasis in true pelvis | |||
| N2: Multiple regional lymph node metastasis in true pelvis | |||
| N3: Metastasis to common iliac lymph nodes | |||
| Distant metastasis (M) | |||
| M0: No distant metastasis | |||
| M1: Distant metastasis | |||
| Clinical staging | |||
| Stage 0a | Ta | N0 | M0 |
| Stage 0is | Tis | N0 | M0 |
| Stage I | T1 | N0 | M0 |
| Stage II | T2a | N0 | M0 |
| T2b | N0 | M0 | |
| Stage III | T3a | N0 | M0 |
| T3b | N0 | M0 | |
| T4a | N0 | M0 | |
| Stage IV | T4b | N0 | M0 |
| Any T | N1 | M0 | |
| Any T | N2 | M0 | |
| Any T | N3 | M0 | |
| Any T | Any N | M1 | |
NON–MUSCLE-INVASIVE BLADDER CANCER
About 70% to 80% of bladder cancers present as non–muscle-invasive tumors. Of these, 60% to 70% are confined to the bladder mucosa (stage Ta), 20% to 30% demonstrate invasion to the subepithelial connective tissue (stage T1), and about 10% present as carcinoma in situ.20,37 The primary treatment for non–muscle-invasive bladder cancer is TURBT, usually followed by immediate instillation of BCG or intravesical chemotherapy (mitomycin C, epirubicin [Ellence], or doxorubicin [Adriamycin]). The decision to instill BCG and/or chemotherapy is based on the risk of cancer progression or recurrence.17,27 A risk calculator is available at http://www.eortc.be/tools/bladdercalculator.
For low-risk tumors (low-grade Ta), TURBT with immediate instillation of chemotherapy is recommended as complete therapy.20,27 Patients with high-risk non–muscle-invasive tumors (high-grade Ta and T1) have about a 50% chance of recurrence with muscle-invasive disease if treated with TURBT alone, and are therefore typically treated with TURBT and intravesical BCG (preferred) or mitomycin C.20,27,32 In patients with high-grade Ta or T1 tumors, 10-year recurrence-free survival after TURBT and BCG immunotherapy is about 80%.29 About one-half of patients with non–muscle-invasive carcinoma in situ will progress to muscle-invasive disease without treatment.20,32 Restaging of high-risk non–muscle-invasive tumors (high-grade Ta, T1, and Tis) with repeat TURBT two to six weeks after diagnosis and preferably before BCG instillation is recommended27,32,38 and is associated with decreased recurrence, with a number needed to treat of 3 to prevent recurrence at three months.39 Patients with high-grade T1 non–muscle-invasive tumors, especially those with high-risk features (e.g., multiple grade 3 T1 tumors with Tis, increased depth of invasion, disease progression with BCG treatment), should be considered for immediate cystectomy.17,20,27
MUSCLE-INVASIVE BLADDER CANCER
Given the aggressive nature of muscle-invasive bladder cancer, timely diagnosis and treatment are crucial.40,41 Radical cystectomy with bilateral pelvic lymphadenectomy and cisplatin-based neoadjuvant chemotherapy are strongly recommended for all patients with resectable nonmetastatic muscle-invasive bladder cancer.36 Five-year survival rates with cystectomy alone are only about 50%; neoadjuvant chemotherapy can improve survival, especially in patients at high risk of progression or recurrence (those with nodal involvement, high-grade tumors, or transmural or vascular invasion).17,20,27,29,36,42–44 When used in conjunction with cystectomy, cisplatin-based neoadjuvant chemotherapy demonstrated an absolute five-year survival benefit of 5% to 8% across several trials, translating to a number needed to treat of 9.29 Segmental (partial) cystectomy and neoadjuvant cisplatin-based chemotherapy may be appropriate for select patients.27,36 Extensive lymph node dissection is associated with improvements in progression-free and overall survival.45 Compared with open surgery, a laparoscopic approach is associated with similar recurrence and survival rates, may reduce pain and the need for blood transfusion, and may speed recovery.46,47 Radical cystectomy improves survival rates compared with external beam radiotherapy and is the preferred treatment for muscle-invasive bladder cancer.48 Radiotherapy may be considered as part of a multimodal bladder-preserving approach or for palliation in patients who are not candidates for cystectomy.17,36
Chemotherapy is the preferred treatment for patients with metastatic disease or unresectable bladder cancer. The two available treatments (combination gemcitabine and cisplatin, and combination methotrexate, vinblastine, doxorubicin, and cisplatin) are associated with a median survival time of about 14 months.17
Overall survival for patients with muscle-invasive bladder cancer is 66% at five years after radical cystectomy and extensive lymph node dissection, and 50% to 60% with bladder preservation therapy.29 Radical cystectomy and reconstruction is associated with 90-day postoperative mortality rates of 3% to 9%,29,50 underscoring the emphasis on quality of life for patients older than 80 years and those with significant comorbidities.
ADJUVANT CHEMOTHERAPY
There is insufficient high-quality evidence to support routine use of adjuvant chemotherapy for patients with bladder cancer. However, chemotherapy may provide benefit in certain high-risk patients.17,51 Platinum-based combination chemotherapy may improve survival in patients with invasive bladder cancer.52 Cisplatin-based chemotherapy improves disease-free survival in those with lymph node involvement.53 Combination gemcitabine and cisplatin is associated with similar overall survival as other regimens, but may be less toxic in patients with unresectable, locally advanced, or metastatic transitional cell bladder cancer.54,55
NONUROTHELIAL BLADDER CANCER
Cystectomy, partial cystectomy, or radiation is the mainstay of treatment for most nonurothelial bladder cancers. Systemic chemotherapy is generally not used for these tumors.27
FOLLOW-UP

| Tumor stage | Follow-up | |
|---|---|---|
| Low-grade Ta | Cystoscopy at three months, then at increasing intervals as appropriate | |
| T1 or high-grade Ta | Cystoscopy and urine cytology every three to six months for two years, then at increasing intervals as appropriate | |
| Consider imaging of the upper urinary tract every one to two years | ||
| Consider testing for urinary tumor markers for urothelial cancer | ||
| Maintenance BCG immunotherapy (preferred if used previously) | ||
| T2 or greater (muscle-invasive disease) | Urine cytology with creatinine and electrolyte levels as clinically indicated | |
| Imaging of chest and cross-sectional imaging of abdomen and pelvis with computed tomography or magnetic resonance imaging every six to 12 months for two to three years, then annually | ||
| After bladder-sparing surgery: | ||
| Urine cytology, creatinine and electrolyte levels, and liver function tests every three to six months for two years, then as clinically indicated | ||
| Imaging as above | ||
| Cystoscopy and urine cytology with or without selected mapping biopsy every three to six months for two years, then at increasing intervals as appropriate | ||
| Consider urethral wash cytology every six to 12 months, especially in patients with carcinoma in situ within bladder or prostatic urethra | ||
| After cystectomy and continent reservoir urinary diversion, vitamin B12 level annually* | ||
Treatment of Recurrent or Persistent Disease
Recurrent non–muscle-invasive bladder cancer is treated with a combination of repeat TURBT, adjuvant intravesical therapy, maintenance BCG immunotherapy, and/or cystectomy, based on tumor and grade.27 For persistent or recurrent muscle-invasive bladder cancer, cystectomy is often recommended for patients with initial bladder preservation. Intravesical BCG immunotherapy may be attempted in patients with low-risk tumors but should be followed with cystectomy if there is no response.27 Chemoradiotherapy or palliative TURBT and supportive care are recommended when recurrent or persistent disease is invasive (T2 or greater).27
Prognosis

| Tumor stage | Five-year survival rate (%) |
|---|---|
| All | 76 |
| In situ | 96 |
| Localized | 70 |
| Regional | 35 |
| Distant | 5 |
| Unknown | 46 |