
Am Fam Physician. 2018;97(5):352-353
Author disclosure: No relevant financial affiliations.
Key Points for Practice
• Alendronate, risedronate, zoledronic acid, and denosumab reduce risk of hip, nonvertebral, and vertebral fracture in women with osteoporosis.
• Pharmacologic treatment should continue for five years.
• Current evidence does not indicate benefit of BMD monitoring during pharmacologic treatment.
• Hormone therapy with estrogen or estrogen/progestogen, or raloxifene should not be used to treat women with osteoporosis.
From the AFP Editors
Osteoporosis, which most commonly occurs in the hip, spine, and wrist, puts persons at increased risk of fragility and fracture. Fragility fractures and low bone mineral density (BMD) as determined by dual-energy x-ray absorptiometry (DEXA) aid in diagnosis. Pharmacologic treatment options, most of which are targeted at preventing bone resorption, include bisphosphonates (i.e., alendronate [Fosamax], risedronate [Actonel], zoledronic acid [Reclast], and ibandronate [Boniva]), peptide hormones (i.e., teriparatide [Forteo] and calcitonin), denosumab (Prolia), calcium, and vitamin D, as well as estrogen and selective estrogen receptor modulators (e.g., raloxifene [Evista]) for postmenopausal women. The American College of Physicians (ACP) has updated its 2008 guideline to include new evidence regarding fracture prevention in adults with low BMD or osteoporosis.
Recommendations
Alendronate, risedronate, zoledronic acid, and denosumab, which have been shown to reduce vertebral, nonvertebral, and hip fractures, should be offered to women with osteoporosis to help decrease their risk of experiencing a hip or vertebral fracture. This recommendation is supported by high-quality evidence of effectiveness in postmenopausal women. Calcium and vitamin D can be added as dietary supplements, but their effectiveness for preventing fractures is not known.
Pharmacologic treatment should continue for five years in these women. Although evidence is insufficient to determine the appropriate duration of pharmacologic treatment, most studies indicate that the benefit lasts up to five years. Continuing treatment after five years may be beneficial for some patients and may be suitable after weighing the risks and benefits of ongoing treatment.
Because current evidence does not indicate benefit of BMD monitoring during the five years of pharmacologic treatment, it does not need to be performed during this time frame. Frequent monitoring is not recommended, because evidence indicates that women with normal DEXA scores do not develop osteoporosis within 15 years.
Hormone therapy with estrogen or estrogen/progestogen, or raloxifene should not be used to treat women with osteoporosis. Although high-quality evidence indicates that raloxifene reduces vertebral fractures in women with osteoporosis, it is not associated with a statistically significant reduction in the risk of nonvertebral or hip fractures and is associated with major harms (e.g., thromboembolism). Based on moderate-quality evidence, there is no difference in fracture rates with estrogen. In addition, estrogen has major harms (e.g., cerebrovascular accidents, venous thromboembolism), which considerably outweigh the possible benefits of its use.
The decision to provide treatment to women 65 years and older with osteopenia who are at high risk of fracture should take into account patient preference, fracture risk, benefits, harms, and costs. In addition, more evidence is needed about use of medications in persons with osteopenia.
Bisphosphonates should be offered to men with osteoporosis to decrease their risk of vertebral fracture. Most trials evaluating men with osteoporosis included women; therefore, specific research in men is needed. No evidence indicates that outcomes of pharmacologic treatment would vary between men and women with similar BMD scores.
Table 1 provides pharmacologic treatment options for low BMD and osteoporosis, and associated adverse effects. Bisphosphonates as a class have been shown to reduce vertebral fractures. In addition, denosumab reduces vertebral, nonvertebral, and hip fractures; teriparatide reduces vertebral and nonvertebral fractures; raloxifene reduces vertebral fractures; and alendronate, risedronate, and zoledronic acid reduce nonvertebral and hip fractures. There is insufficient evidence, however, to indicate that one medication is superior to another for preventing fractures.
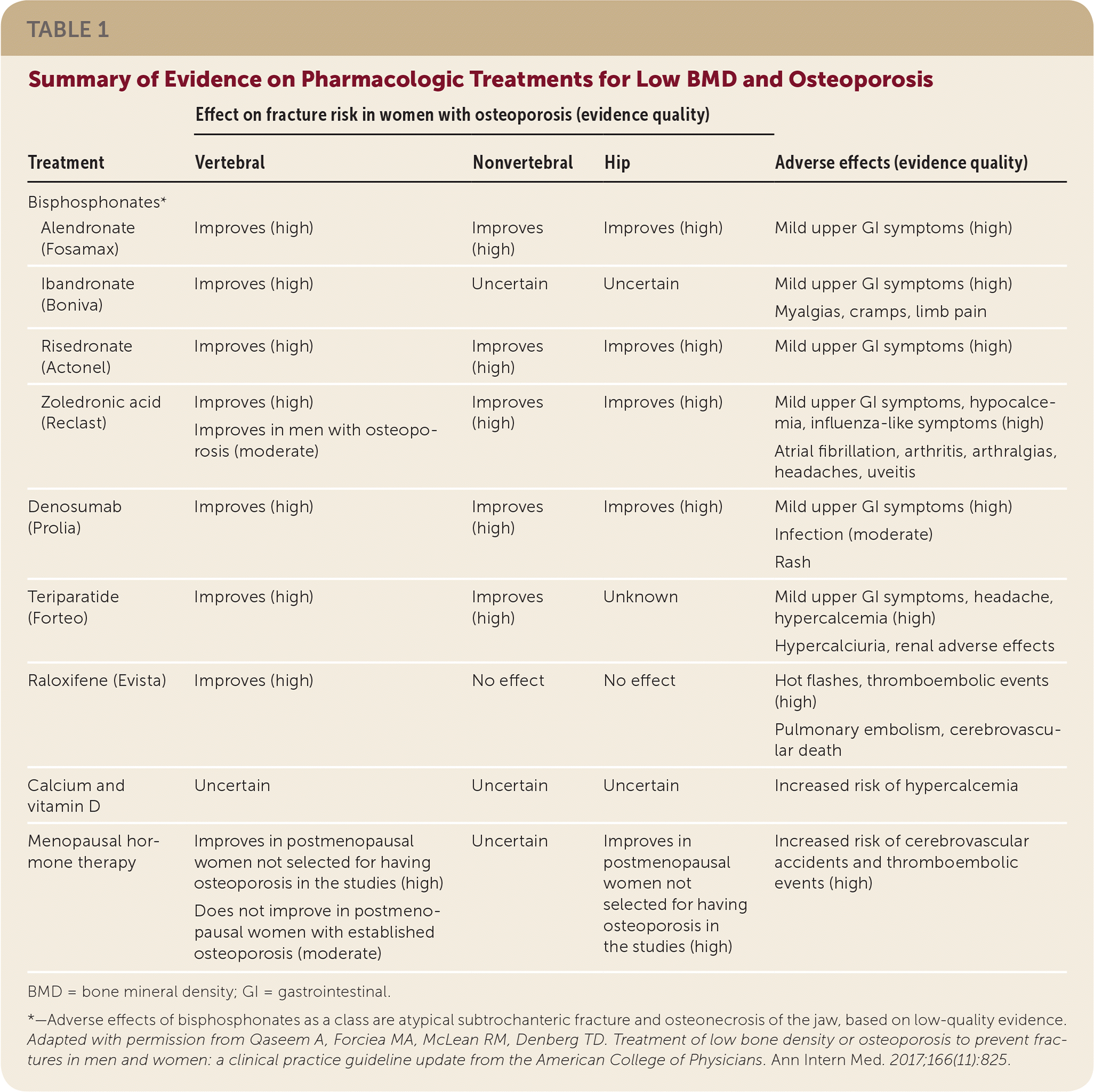
| Treatment | Effect on fracture risk in women with osteoporosis (evidence quality) | Adverse effects (evidence quality) | |||
|---|---|---|---|---|---|
| Vertebral | Nonvertebral | Hip | |||
| Bisphosphonates* | |||||
| Alendronate (Fosamax) | Improves (high) | Improves (high) | Improves (high) | Mild upper GI symptoms (high) | |
| Ibandronate (Boniva) | Improves (high) | Uncertain | Uncertain | Mild upper GI symptoms (high) Myalgias, cramps, limb pain | |
| Risedronate (Actonel) | Improves (high) | Improves (high) | Improves (high) | Mild upper GI symptoms (high) | |
| Zoledronic acid (Reclast) | Improves (high) | Improves (high) | Improves (high) | Mild upper GI symptoms, hypocalcemia, influenza-like symptoms (high) | |
| Improves in men with osteoporosis (moderate) | Atrial fibrillation, arthritis, arthralgias, headaches, uveitis | ||||
| Denosumab (Prolia) | Improves (high) | Improves (high) | Improves (high) | Mild upper GI symptoms (high) | |
| Infection (moderate) | |||||
| Rash | |||||
| Teriparatide (Forteo) | Improves (high) | Improves (high) | Unknown | Mild upper GI symptoms, headache, hypercalcemia (high) | |
| Hypercalciuria, renal adverse effects | |||||
| Raloxifene (Evista) | Improves (high) | No effect | No effect | Hot flashes, thromboembolic events (high) | |
| Pulmonary embolism, cerebrovascular death | |||||
| Calcium and vitamin D | Uncertain | Uncertain | Uncertain | Increased risk of hypercalcemia | |
| Menopausal hormone therapy | Improves in postmenopausal women not selected for having osteoporosis in the studies (high) | Uncertain | Improves in postmenopausal women not selected for having osteoporosis in the studies (high) | Increased risk of cerebrovascular accidents and thromboembolic events (high) | |
| Does not improve in postmenopausal women with established osteoporosis (moderate) | |||||
Guideline source: American College of Physicians
Evidence rating system used? Yes
Systematic literature search described? Yes
Guideline developed by participants without relevant financial ties to industry? Yes
Recommendations based on patient-oriented outcomes? Yes
Published source:Ann Intern Med. June 6, 2017;166(11):818–839
Endorsed by the AAFP, April 2017:http://www.aafp.org/patient-care/clinical-recommendations/all/osteoporosis-cpg.html
LISA HAUK
AFP Senior Associate Editor
