Am Fam Physician. 2019;100(7):403-405
Author disclosure: No relevant financial affiliations.
Clinical Question
Does treatment of mild to moderate hypertension in pregnancy with antihypertensive drugs improve pregnancy outcomes?
Evidence-Based Answer
Compared with placebo, antihypertensive drug therapy for mild to moderate hypertension (defined by the authors as a blood pressure of 140 to 169 mm Hg systolic or 90 to 109 mm Hg diastolic) caused by chronic hypertension, gestational hypertension, or preeclampsia during pregnancy does not affect any pregnancy outcomes. However, it does reduce the risk of developing severe hypertension (relative risk [RR] = 0.49; 95% CI, 0.40 to 0.60; number needed to treat [NNT] = 10). Beta blockers and calcium channel blockers are more effective than methyldopa in preventing severe hypertension (NNT = 26).1 (Strength of Recommendation: A, based on consistent, good-quality patient-oriented evidence.)
Practice Pointers
In the United States, approximately 1% of pregnant women have chronic hypertension. Women with chronic hypertension in pregnancy have a higher risk of developing adverse obstetric outcomes, including gestational diabetes mellitus, postpartum hemorrhage, fetal growth restriction, preterm birth, and neonatal death, as well as a higher risk of preeclampsia. Preeclampsia occurs in 2% to 8% of all pregnancies worldwide.1,2 The authors of this review sought to determine the effect of treating pregnant women with mildly to moderately elevated blood pressure caused by hypertension, gestational hypertension, or preeclampsia.
This Cochrane review included 58 randomized controlled trials involving 5,909 women.1 The overall quality of the studies included in the review was rated as moderate to poor by the authors, mostly because of a higher risk of performance and detections bias. Sixteen of the included studies were published after 2000, and the largest featured 314 women. High-income countries as well as middle- and low-income countries were well represented. Thirty-one trials (n = 3,485) compared antihypertensive drug therapies with placebo or no therapy, and 29 trials (n = 2,774) compared one antihypertensive drug with another.
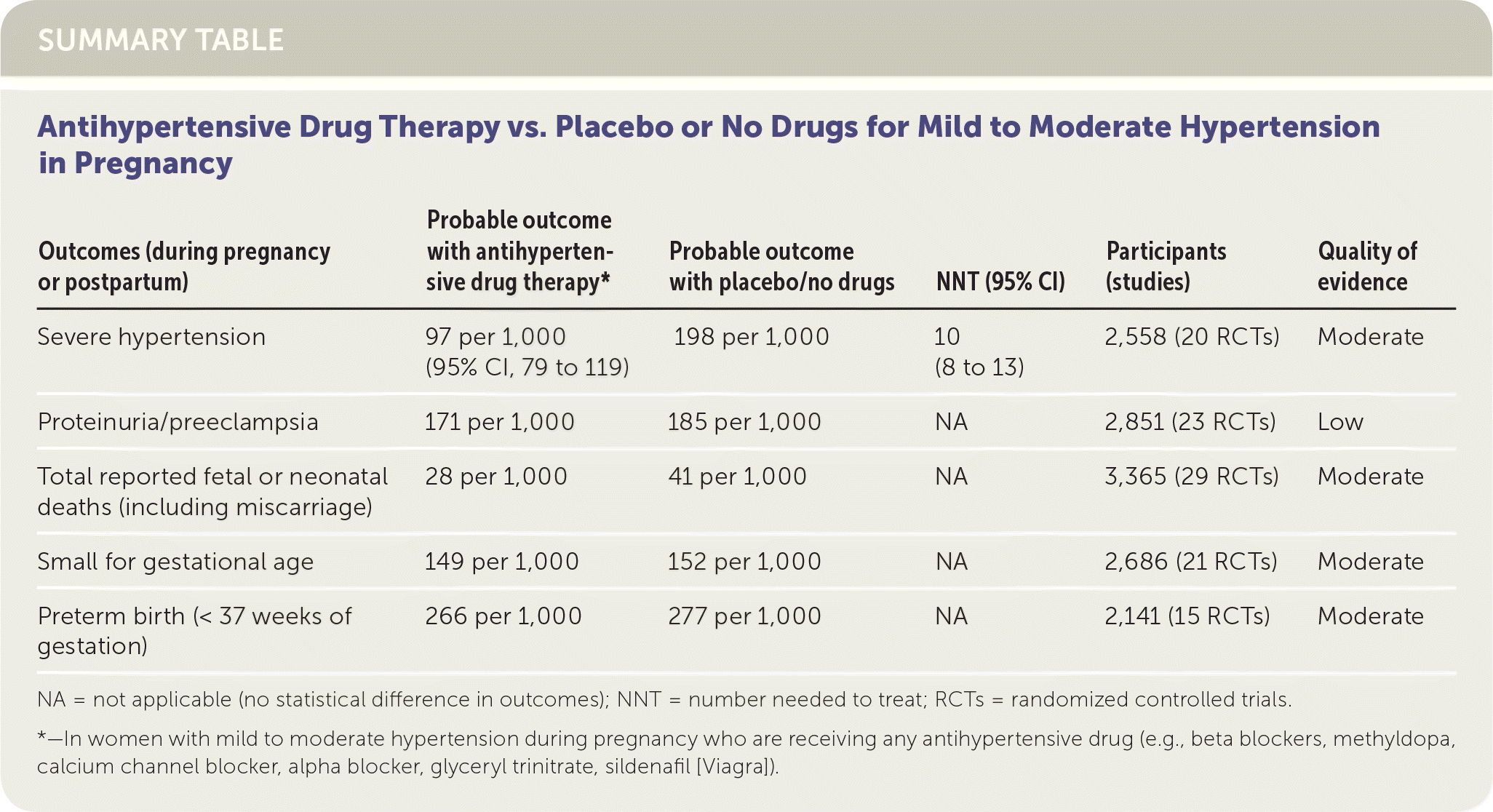
This review included women with any form of hypertension in pregnancy (chronic, gestational, or preeclampsia) with mild to moderate elevations in blood pressure. The primary outcomes examined in the analysis of antihypertensive therapy vs. placebo were development of severe hypertension in pregnancy (in general, greater than 170/110 mm Hg, although the authors also included trials in which severe hypertension was defined as greater than 160 mm Hg systolic), development of proteinuria/preeclampsia, miscarriage and fetal/neonatal death, preterm birth (at less than 37 weeks of gestation), and infants small for gestational age. There was a significant decrease in the development of severe hypertension, again generally defined by the authors as systolic pressure greater than 170 mm Hg or diastolic pressure greater than 110 mm Hg in the antihypertensive therapy group (RR = 0.49; 95% CI, 0.40 to 0.60; NNT = 10). There was no difference in any of the other primary outcomes. Of the numerous secondary outcomes discussed, only one was significant: treatment of mild to moderate hypertension resulted in a decreased risk of neonatal respiratory distress (absolute risk reduction [ARR] = 0.53; 95% CI, 0.2 to 0.99).
In the analysis of trials comparing one antihypertensive drug with another, no statistically significant difference in outcomes could be demonstrated. The majority of studies used medication classes commonly prescribed in the United States during pregnancy (beta blockers, calcium channel blockers, or methyldopa), although other medications (i.e., vasodilators, ketanserin, glyceryl trinitrate, furosemide [Lasix], sildenafil [Viagra]) were represented. Patients treated with calcium channel blockers or beta blockers were less likely to develop severe hypertension than those treated with methyldopa (RR = 0.70; 95% CI, 0.59 to 0.93). With regard to secondary outcomes, women treated with beta blockers or calcium channel blockers were less likely than those treated with methyldopa to have a cesarean delivery (ARR = 0.84; 95% CI, 0.74 to 0.95). Treatment of mild to moderate hypertension did not result in any other outcome differences or adverse effects.
The American College of Obstetricians and Gynecologists,2 Hypertension Canada/the Society of Obstetricians and Gynaecologists of Canada,3 and the European Society of Cardiology/European Society of Hypertension4 all recommend pharmacologic treatment of severe hypertension in pregnancy (which they define as systolic blood pressure of 160 mm Hg or greater or diastolic blood pressure of 110 mm Hg or greater). Guidelines for the treatment of mild to moderate hypertension in pregnancy differ. The American College of Obstetricians and Gynecologists recommends that antihypertensive drug therapy be initiated only for systolic blood pressure of 160 mm Hg or greater or diastolic blood pressure of 110 mm Hg or greater, with a goal treatment range of 120 to 159/80 to 109 mm Hg. Hypertension Canada/the Society of Obstetricians and Gynaecologists of Canada guidelines recommend antihypertensive drug therapy for an average systolic measurement of at least 140 mm Hg or diastolic measurement of at least 90 mm Hg with a diastolic target of 85 mm Hg. The European Society of Cardiology/European Society of Hypertension 2018 hypertension guidelines recommend treatment for blood pressure greater than 150/95 mm Hg. The National Institute for Health and Clinical Excellence guidelines, which were last released in 2010, recommended that blood pressure be maintained at less than 150/100 mm Hg, but they are currently being updated.5
| Outcomes (during pregnancy or postpartum) | Probable outcome with antihypertensive drug therapy* | Probable outcome with placebo/no drugs | NNT (95% CI) | Participants (studies) | Quality of evidence |
|---|---|---|---|---|---|
| Severe hypertension | 97 per 1,000 (95% CI, 79 to 119) | 198 per 1,000 | 10 (8 to 13) | 2,558 (20 RCTs) | Moderate |
| Proteinuria/preeclampsia | 171 per 1,000 | 185 per 1,000 | NA | 2,851 (23 RCTs) | Low |
| Total reported fetal or neonatal deaths (including miscarriage) | 28 per 1,000 | 41 per 1,000 | NA | 3,365 (29 RCTs) | Moderate |
| Small for gestational age | 149 per 1,000 | 152 per 1,000 | NA | 2,686 (21 RCTs) | Moderate |
| Preterm birth (< 37 weeks of gestation) | 266 per 1,000 | 277 per 1,000 | NA | 2,141 (15 RCTs) | Moderate |
The practice recommendations in this activity are available at http://www.cochrane.org/CD002252.
Editor's Note: The numbers needed to treat reported in this Cochrane for Clinicians were calculated by the authors based on raw data provided in the original Cochrane review.