
Am Fam Physician. 2020;101(4):233-238
Related Putting Prevention into Practice: Risk Assessment, Genetic Counseling, and Genetic Testing for BRCA-Related Cancer: Recommendation Statement
As published by the USPSTF.
Summary of Recommendation and Evidence
The USPSTF recommends that primary care clinicians assess women with a personal or family history of breast, ovarian, tubal, or peritoneal cancer or who have an ancestry associated with breast cancer susceptibility 1 and 2 (BRCA1/2) gene mutations with an appropriate brief familial risk assessment tool. Women with a positive result on the risk assessment tool should receive genetic counseling and, if indicated after counseling, genetic testing (Table 1). B recommendation.
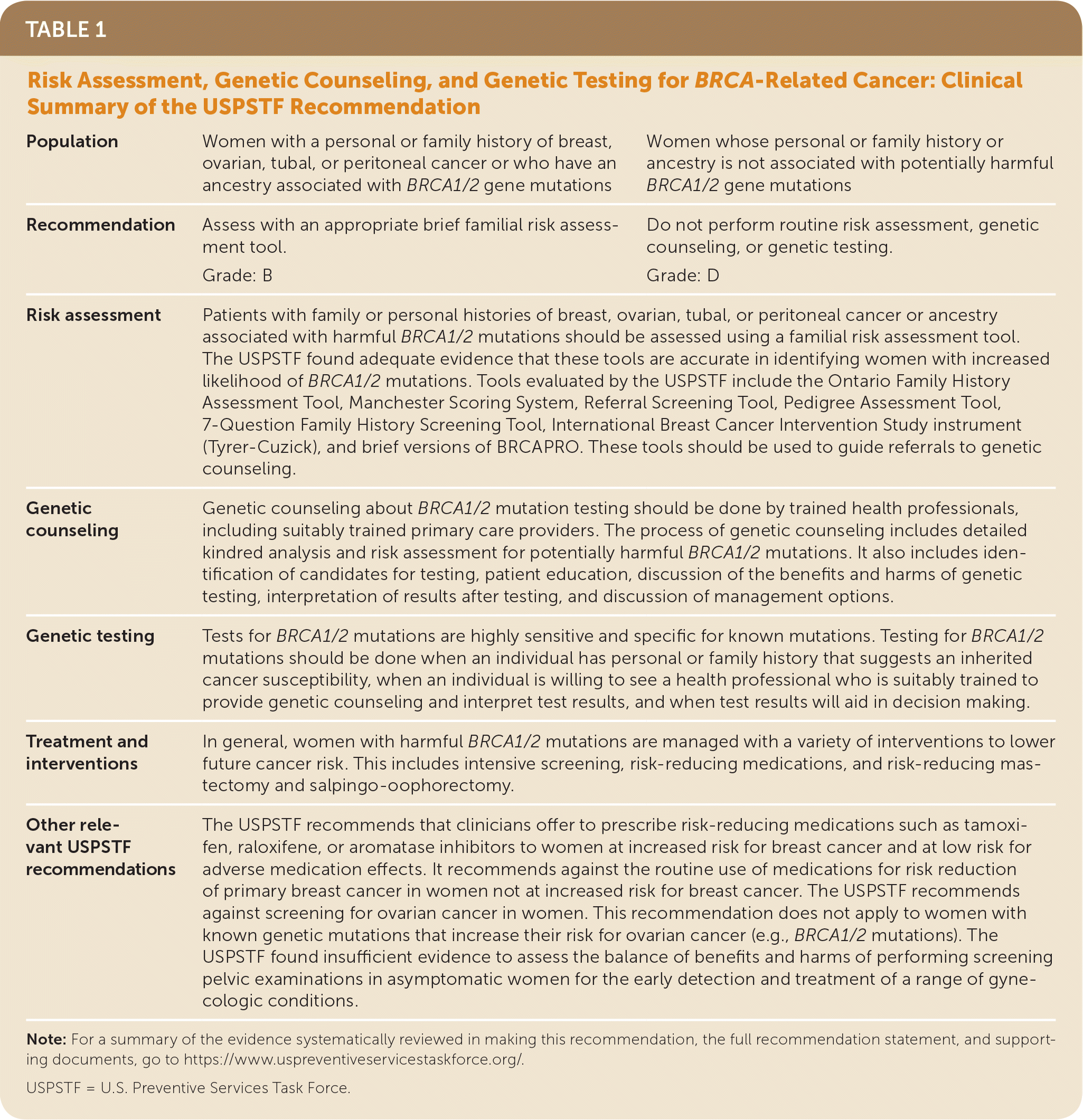
| Population | Women with a personal or family history of breast, ovarian, tubal, or peritoneal cancer or who have an ancestry associated with BRCA1/2 gene mutations | Women whose personal or family history or ancestry is not associated with potentially harmful BRCA1/2 gene mutations |
| Recommendation | Assess with an appropriate brief familial risk assessment tool. | Do not perform routine risk assessment, genetic counseling, or genetic testing. |
| Grade: B | Grade: D | |
| Risk assessment | Patients with family or personal histories of breast, ovarian, tubal, or peritoneal cancer or ancestry associated with harmful BRCA1/2 mutations should be assessed using a familial risk assessment tool. The USPSTF found adequate evidence that these tools are accurate in identifying women with increased likelihood of BRCA1/2 mutations. Tools evaluated by the USPSTF include the Ontario Family History Assessment Tool, Manchester Scoring System, Referral Screening Tool, Pedigree Assessment Tool, 7-Question Family History Screening Tool, International Breast Cancer Intervention Study instrument (Tyrer-Cuzick), and brief versions of BRCAPRO. These tools should be used to guide referrals to genetic counseling. | |
| Genetic counseling | Genetic counseling about BRCA1/2 mutation testing should be done by trained health professionals, including suitably trained primary care providers. The process of genetic counseling includes detailed kindred analysis and risk assessment for potentially harmful BRCA1/2 mutations. It also includes identification of candidates for testing, patient education, discussion of the benefits and harms of genetic testing, interpretation of results after testing, and discussion of management options. | |
| Genetic testing | Tests for BRCA1/2 mutations are highly sensitive and specific for known mutations. Testing for BRCA1/2 mutations should be done when an individual has personal or family history that suggests an inherited cancer susceptibility, when an individual is willing to see a health professional who is suitably trained to provide genetic counseling and interpret test results, and when test results will aid in decision making. | |
| Treatment and interventions | In general, women with harmful BRCA1/2 mutations are managed with a variety of interventions to lower future cancer risk. This includes intensive screening, risk-reducing medications, and risk-reducing mastectomy and salpingo-oophorectomy. | |
| Other relevant USPSTF recommendations | The USPSTF recommends that clinicians offer to prescribe risk-reducing medications such as tamoxifen, raloxifene, or aromatase inhibitors to women at increased risk for breast cancer and at low risk for adverse medication effects. It recommends against the routine use of medications for risk reduction of primary breast cancer in women not at increased risk for breast cancer. The USPSTF recommends against screening for ovarian cancer in women. This recommendation does not apply to women with known genetic mutations that increase their risk for ovarian cancer (e.g., BRCA1/2 mutations). The USPSTF found insufficient evidence to assess the balance of benefits and harms of performing screening pelvic examinations in asymptomatic women for the early detection and treatment of a range of gynecologic conditions. | |
The USPSTF recommends against routine risk assessment, genetic counseling, or genetic testing for women whose personal or family history or ancestry is not associated with potentially harmful BRCA1/2 gene mutations. D recommendation.
Rationale
IMPORTANCE
Potentially harmful mutations of the BRCA1/2 genes are associated with increased risk for breast, ovarian, fallopian tube, and peritoneal cancer.1–6 For women in the United States, breast cancer is the most common cancer after nonmelanoma skin cancer and the second leading cause of cancer death.7 In the general population, BRCA1/2 mutations occur in an estimated 1 in 300 to 500 women and account for 5% to 10% of breast cancer cases and 15% of ovarian cancer cases.8–11 A woman’s risk for breast cancer increases if she has clinically significant mutations in the BRCA1/2 genes.12,13 Mutations in the BRCA1/2 genes increase breast cancer risk by 45% to 65% by age 70 years. Risk of ovarian, fallopian tube, or peritoneal cancer increases to 39% for BRCA1 mutations and 10% to 17% for BRCA2 mutations.12,13
DETECTION
Genetic risk assessment and BRCA1/2 mutation testing is a multistep process that begins with identifying patients with family or personal histories of breast, ovarian, tubal, or peritoneal cancer; family members with known harmful BRCA1/2 mutations; or ancestry associated with harmful BRCA1/2 mutations. Risk for clinically significant BRCA1/2 mutations can be further evaluated with genetic counseling by suitably trained health care clinicians, followed by genetic testing of selected high-risk individuals and posttest counseling about results. The USPSTF found adequate evidence that familial risk assessment tools are accurate in identifying women with increased likelihood of BRCA1/2 mutations. These tools can be used by primary care clinicians to guide referrals to genetic counseling.
The USPSTF has previously established that there is adequate evidence that current genetic tests can accurately detect known BRCA1/2 mutations.14
BENEFITS OF SCREENING, GENETIC COUNSELING, AND GENETIC TESTING
The USPSTF found adequate evidence that the benefits of risk assessment, genetic counseling, and genetic testing are moderate in women whose family history is associated with an increased risk for harmful mutations in the BRCA1/2 genes.
The USPSTF found adequate evidence that the benefits of risk assessment, genetic counseling, and genetic testing are small to none in women whose family history is not associated with an increased risk for harmful mutations in the BRCA1/2 genes.
HARMS OF SCREENING, GENETIC COUNSELING, AND GENETIC TESTING
The USPSTF found adequate evidence that the harms associated with risk assessment, genetic counseling, genetic testing, and interventions are small to moderate.
USPSTF ASSESSMENT
The USPSTF concludes with moderate certainty that the net benefit of risk assessment for increased risk of BRCA1/2 mutations, testing for BRCA1/2 mutations, and use of risk-reducing interventions outweighs the harms in women whose family or personal history is associated with an increased risk for potentially harmful mutations in the BRCA1/2 genes.
The USPSTF concludes with moderate certainty that the harms of risk assessment for increased risk of BRCA1/2 mutations, testing for BRCA1/2 mutations, and use of risk-reducing interventions outweigh the benefits in women whose family or personal history is not associated with an increased risk for potentially harmful mutations in the BRCA1/2 genes.
Clinical Considerations
PATIENT POPULATION UNDER CONSIDERATION
This recommendation applies to women who are asymptomatic for BRCA-related cancer and have unknown BRCA mutation status. It includes women who have never been diagnosed with BRCA-related cancer, as well as those with a previous breast, ovarian, tubal, or peritoneal cancer diagnosis who have completed treatment and are considered cancer free but have not been previously tested. While this recommendation applies to women, the net benefit estimates are driven by biological sex (i.e., male/female) rather than gender identity. Persons should consider their sex at birth to determine which recommendation best applies to them.
ASSESSMENT OF RISK
Mutations in the BRCA1/2 genes cluster in families, showing an autosomal dominant pattern of inheritance in either the mother’s or father’s family. When taking medical and family history information from patients, primary care clinicians should ask about specific types of cancer, primary cancer sites, which family members were affected, and whether relatives had multiple types of primary cancer. Clinicians should also inquire about the age at diagnosis, age at death, and sex of affected family members, both immediate (i.e., parents and siblings) as well as more distant (i.e., aunts, uncles, grandparents, and cousins).
For women who have family members with breast, ovarian, tubal, or peritoneal cancer or have a personal history of these types of cancer, primary care clinicians may use appropriate brief familial risk assessment tools to determine the need for in-depth genetic counseling. Tools evaluated by the USPSTF include the Ontario Family History Assessment Tool (Table 215–18), Manchester Scoring System (Table 316,18–21), Referral Screening Tool (Table 422), Pedigree Assessment Tool (Table 523,24), 7-Question Family History Screening Tool (Table 625,26), International Breast Cancer Intervention Study instrument (Tyrer-Cuzick) (Table 726,27), and brief versions of BRCAPRO. Each of these tools has been validated and accurately estimates the likelihood of carrying a harmful BRCA1/2 mutation. They can be used to guide referrals to genetic counseling for more definitive risk assessment.28 General breast cancer risk assessment models (e.g., the National Cancer Institute Breast Cancer Risk Assessment Tool, which is based on the Gail model) are not designed to identify BRCA-related cancer risk and should not be used for this purpose.

| Risk factor | Points |
|---|---|
| Breast and ovarian cancer | |
| Mother | 10 |
| Sibling | 7 |
| Second-/third-degree relative | 5 |
| Breast cancer relatives | |
| Parent | 4 |
| Sibling | 3 |
| Second-/third-degree relative | 2 |
| Male relative (add to above) | 2 |
| Breast cancer characteristics | |
| Onset age, y | |
| 20–29 | 6 |
| 30–39 | 4 |
| 40–49 | 2 |
| Premenopausal/perimenopausal | 2 |
| Bilateral/multifocal | 3 |
| Ovarian cancer relatives | |
| Mother | 7 |
| Sibling | 4 |
| Second-/third-degree relative | 3 |
| Ovarian cancer onset, y | |
| < 40 | 6 |
| 40–60 | 4 |
| > 60 | 2 |
| Prostate cancer onset | |
| Age < 50 y | 1 |
| Colon cancer onset | |
| Age < 50 y | 1 |
| Family total: | ______ |
| Referral† | ≥10 |

| Risk factor (age at onset for relative in direct lineage) | BRCA1 score | BRCA2 score |
|---|---|---|
| Female breast cancer, y | ||
| <30 | 6 | 5 |
| 30–39 | 4 | 4 |
| 40–49 | 3 | 3 |
| 50–59 | 2 | 2 |
| ≥60 | 1 | 1 |
| Male breast cancer, y | ||
| <60 | 5‡ | 8§ |
| ≥60 | 5‡ | 5§ |
| Ovarian cancer, y | ||
| <60 | 8 | 5 |
| ≥60 | 5 | 5 |
| Pancreatic cancer | ||
| Any age | 0 | 1 |
| Prostate cancer, y | ||
| <60 | 0 | 2 |
| ≥60 | 0 | 1 |
| Total individual genes | 10 | 10 |
| Total for combined = 15 | ||

| History of breast or ovarian cancer in the family? If yes, complete checklist. | ||
|---|---|---|
| Risk factor | Breast cancer at age ≤50 y | Ovarian cancer at any age |
| Yourself | □ | □ |
| Mother | □ | □ |
| Sister | □ | □ |
| Daughter | □ | □ |
| Mother’s side | ||
| Grandmother | □ | □ |
| Aunt | □ | □ |
| Father’s side | ||
| Grandmother | □ | □ |
| Aunt | □ | □ |
| ≥2 cases of breast cancer after age 50 y on same side of family | □ | □ |
| Male breast cancer at any age in any relative | □ | □ |
| Jewish ancestry | □ | □ |
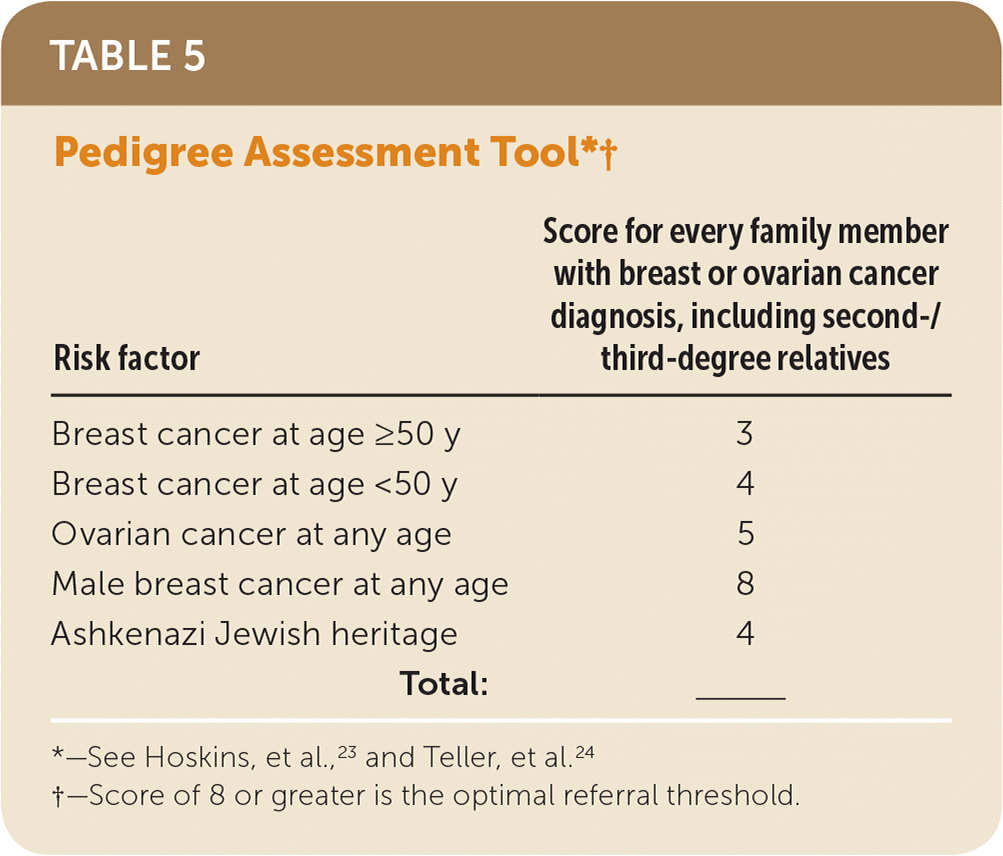
| Risk factor | Score for every family member with breast or ovarian cancer diagnosis, including second-/third-degree relatives |
|---|---|
| Breast cancer at age ≥50 y | 3 |
| Breast cancer at age <50 y | 4 |
| Ovarian cancer at any age | 5 |
| Male breast cancer at any age | 8 |
| Ashkenazi Jewish heritage | 4 |
| Total: | ______ |

| No. | Questions |
|---|---|
| 1 | Did any of your first-degree relatives have breast or ovarian cancer? |
| 2 | Did any of your relatives have bilateral breast cancer? |
| 3 | Did any man in your family have breast cancer? |
| 4 | Did any woman in your family have breast and ovarian cancer? |
| 5 | Did any woman in your family have breast cancer before age 50 y? |
| 6 | Do you have 2 or more relatives with breast and/or ovarian cancer? |
| 7 | Do you have 2 or more relatives with breast and/or bowel cancer? |
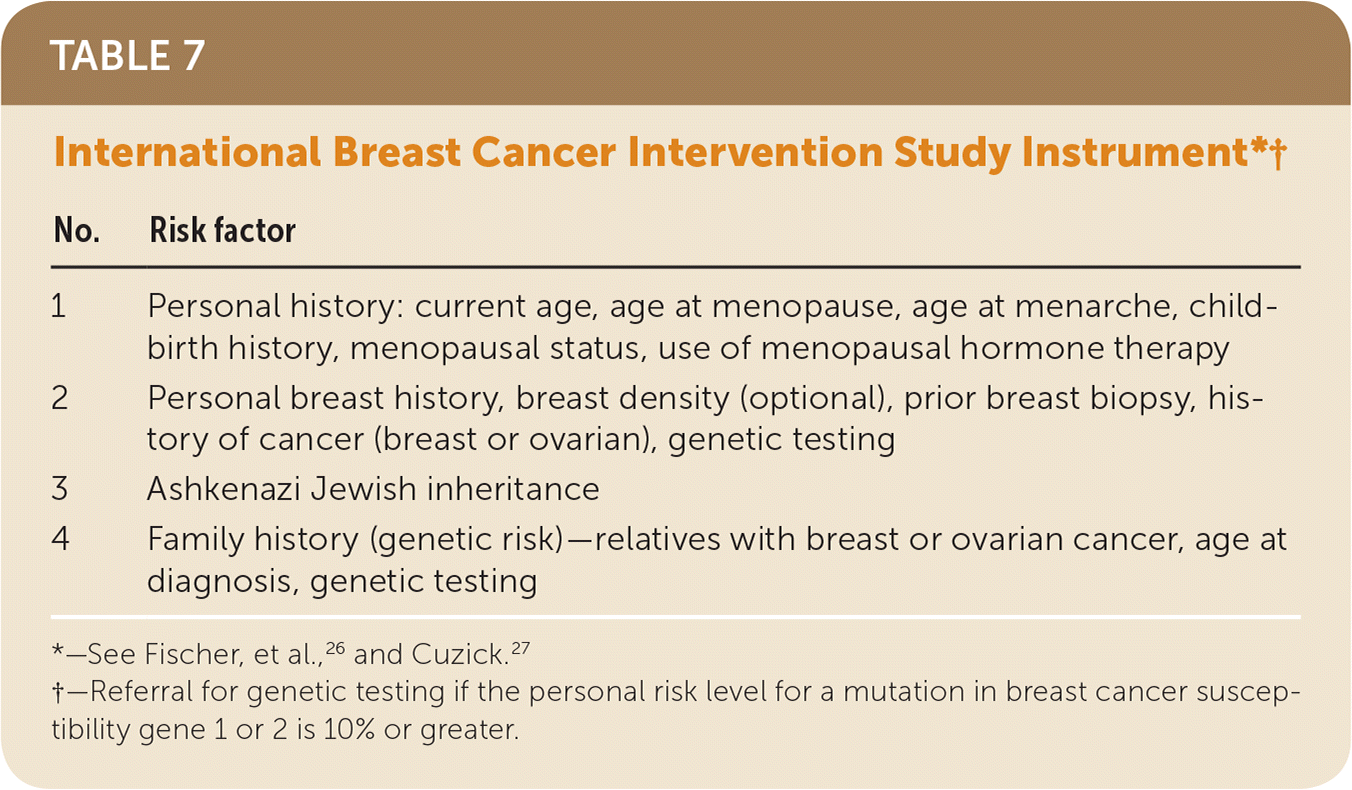
| No. | Risk factor |
|---|---|
| 1 | Personal history: current age, age at menopause, age at menarche, child-birth history, menopausal status, use of menopausal hormone therapy |
| 2 | Personal breast history, breast density (optional), prior breast biopsy, history of cancer (breast or ovarian), genetic testing |
| 3 | Ashkenazi Jewish inheritance |
| 4 | Family history (genetic risk)—relatives with breast or ovarian cancer, age at diagnosis, genetic testing |
In general, these brief familial risk assessment tools include factors associated with increased likelihood of potentially harmful BRCA1/2 mutations. These include breast cancer diagnosis before age 50 years, bilateral breast cancer, presence of both breast and ovarian cancer in one individual, male family members with breast cancer, multiple cases of breast cancer in the family, 1 or more family members with 2 primary types of BRCA-related cancer (such as ovarian cancer), and Ashkenazi Jewish ancestry. The USPSTF recognizes that each risk assessment tool has advantages and limitations and found insufficient evidence to recommend one over another.
GENETIC COUNSELING
The process of genetic counseling includes detailed kindred analysis and risk assessment for potentially harmful BRCA1/2 mutations. It also includes identification of candidates for testing, patient education, discussion of the benefits and harms of genetic testing, interpretation of results after testing, and discussion of management options. Genetic counseling about BRCA1/2 mutation testing should be performed by trained health professionals, including suitably trained primary care clinicians. Several professional organizations describe the skills and training necessary to provide comprehensive genetic counseling.
GENETIC TESTING
Testing for BRCA1/2 mutations should be performed only when an individual has personal or family history that suggests an inherited cancer susceptibility, when an individual is willing to talk with a health professional who is suitably trained to provide genetic counseling and interpret test results, and when test results will aid in decision-making. Clinical practice guidelines recommend that BRCA1/2 mutation testing begin with a relative with known BRCA-related cancer, including male relatives, to determine if a clinically significant mutation is detected in the family before testing individuals without cancer.29 If an affected family member with a BRCA-related cancer is not available, then the relative with the highest probability of mutation should be tested. The type of mutation analysis required depends on family history. Individuals from families with known mutations or from ancestry groups in which certain mutations are more common (e.g., Ashkenazi Jewish founder mutations) can be tested for these specific mutations. Because risk assessment is primarily based on family history, it is unclear how women with a limited or unknown family history should be assessed for BRCA1/2 mutation risk and potential referral to counseling or genetic testing.
Tests for BRCA1/2 mutations are highly sensitive and specific for known mutations. The availability of testing options has changed since the 2013 U.S. Supreme Court ruling that determined human genes are not patentable (Association for Molecular Pathology v Myriad Genetics, Inc.).30 Previously, BRCA1/2 mutation testing in the United States was mainly conducted by 1 laboratory. Since the ruling, the number of testing options has significantly increased, with more than 80 multigene panels that include BRCA1/2, as well as tests marketed directly to consumers.31
Guidelines from the American College of Medical Genetics and Genomics, which were updated in 2015, recommend new standard terminology for reporting BRCA1/2 mutations identified by genetic tests. These include a 5-tier terminology system using the terms “pathogenic,” “likely pathogenic,” “uncertain significance,” “likely benign,” and “benign.”32
TREATMENT AND INTERVENTIONS
Management of increased cancer risk related to BRCA1/2 mutations is beyond the scope of this Recommendation Statement. In general, care for women with harmful BRCA1/2 mutations consists of a variety of interventions to lower future cancer risk. This includes intensive screening, risk-reducing medications, and risk-reducing mastectomy and salpingo-oophorectomy.
ADDITIONAL TOOLS AND RESOURCES
The National Cancer Institute Cancer Genetics Services Directory provides a list of professionals who offer services related to cancer genetics, including cancer risk assessment, genetic counseling, and genetic testing.33
This recommendation statement was first published in JAMA. 2019;322(7):652–665.
The “Other Related USPSTF Recommendation,” Other Considerations,” “Discussion,” “Update of Previous USPSTF Recommendation,” and “Recommendations of Others” sections of this recommendation statement are available at https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/brca-related-cancer-risk-assessment-genetic-counseling-and-genetic-testing1.
The USPSTF recommendations are independent of the U.S. government. They do not represent the views of the Agency for Healthcare Research and Quality, the U.S. Department of Health and Human Services, or the U.S. Public Health Service.
