
Am Fam Physician. 2020;101(12):730-738
Author disclosure: No relevant financial affiliations.
Acute respiratory distress syndrome (ARDS) is noncardiogenic pulmonary edema that manifests as rapidly progressive dyspnea, tachypnea, and hypoxemia. Diagnostic criteria include onset within one week of a known insult or new or worsening respiratory symptoms, profound hypoxemia, bilateral pulmonary opacities on radiography, and inability to explain respiratory failure by cardiac failure or fluid overload. ARDS is thought to occur when a pulmonary or extrapulmonary insult causes the release of inflammatory mediators, promoting inflammatory cell accumulation in the alveoli and microcirculation of the lung. Inflammatory cells damage the vascular endothelium and alveolar epithelium, leading to pulmonary edema, hyaline membrane formation, decreased lung compliance, and decreased gas exchange. Most cases are associated with pneumonia or sepsis. ARDS is responsible for one in 10 admissions to intensive care units and one in four mechanical ventilations. In-hospital mortality for patients with severe ARDS ranges from 46% to 60%. ARDS often must be differentiated from pneumonia and congestive heart failure, which typically has signs of fluid overload. Treatment of ARDS is supportive and includes mechanical ventilation, prophylaxis for stress ulcers and venous thromboembolism, nutritional support, and treatment of the underlying injury. Low tidal volume and high positive end-expiratory pressure improve outcomes. Prone positioning is recommended for some moderate and all severe cases. As patients with ARDS improve and the underlying illness resolves, a spontaneous breathing trial is indicated to assess eligibility for ventilator weaning. Patients who survive ARDS are at risk of diminished functional capacity, mental illness, and decreased quality of life; ongoing care by a primary care physician is beneficial for these patients.
Acute respiratory distress syndrome (ARDS) is a rapidly progressive noncardiogenic pulmonary edema that initially manifests as dyspnea, tachypnea, and hypoxemia, then quickly evolves into respiratory failure. Because roughly one-half of intensive care units (ICUs) in the United States are not staffed with intensivists,1 many family physicians care for patients with ARDS. In addition, family physicians may take on the role of ICU physicians during times of unprecedented threats to the health care system and severe resource shortages.2
The Berlin criteria used for the diagnosis of ARDS in adults.3 These criteria are based on timing of symptom onset (within one week of known clinical insult or new or worsening respiratory symptoms); bilateral opacities on chest imaging that are not fully explained by effusions, lobar or lung collapse, or nodules; the likely source of pulmonary edema (respiratory failure not fully explained by cardiac failure or fluid overload); and oxygenation as measured by the ratio of partial pressure of arterial oxygen (Pao2) to fraction of inspired oxygen (Fio2). ARDS is classified as mild, moderate, or severe based on the following criteria:
Mild: 200 mm Hg < Pao2/Fio2 ratio ≤ 300 mm Hg with positive end-expiratory pressure (PEEP) or continuous positive airway pressure ≥ 5 cm H2O.
Moderate: 100 mm Hg < Pao2/Fio2 ratio ≤ 200 mm Hg with PEEP ≥ 5 cm H2O.
Severe: Pao2/Fio2 ratio ≤ 100 mm Hg with PEEP ≥ 5 cm H2O.
The Pediatric Acute Lung Injury Consensus Conference criteria for ARDS in children are similar to the Berlin criteria but allow for other measures of oxygenation, and they address preexisting lung and heart disease (see Figure 1 at https://bit.ly/2WcFiQy).4
Pathophysiology
ARDS progresses through several phases after a direct pulmonary or indirect extrapulmonary insult. In the exudative phase, which may last seven to 10 days, alveolar macrophages secrete mediators that lead to accumulation of inflammatory cells in the lung. This accumulation, in combination with neutrophil and alveolar epithelial cell activation, leads to tissue injury. Inflammatory cells migrate across the vascular endothelial and alveolar epithelial surfaces and proinflammatory mediators and chemokines are released, leading to pathologic vascular permeability, gaps in the alveolar epithelial barrier, and necrosis of types I and II alveolar cells. Intravascular coagulation in the alveolar capillaries leads to micro-thrombi. The end result of these changes is pulmonary edema, loss of surfactant, and deposition of dead cells and debris along the alveoli (hyaline membranes), which decrease pulmonary compliance and make gas exchange difficult.5–7 Chest radiography may show changes from air space opacification to coarse reticular opacification during this phase 8 (Figure 1).

The proliferative phase begins the process of lung repair over the next two to three weeks. Anti-inflammatory cytokines deactivate inciting neutrophils, which then undergo apoptosis and phagocytosis. Type II alveolar cells proliferate and differentiate into type I cells, reestablishing the integrity of the epithelial lining. Alveolar ion channels and aquaporins are reexpressed, drawing fluid out of the alveoli and into the pulmonary microcirculation and lung lymphatics. Simultaneously, alveolar cells and macrophages remove debris from the alveoli, and endothelial cells reestablish vascular integrity, allowing the lungs to recover.5–7
The fibrotic phase, which does not occur in all patients, is characterized by ongoing inflammation, extensive basement membrane damage, persistent edema, intra-alveolar and interstitial fibrosis, and microvascular damage. Shear forces associated with mechanical ventilation may promote the development of the fibrotic phase, although lung protective ventilation is thought to ameliorate this effect.9 Progression to the fibrotic phase is associated with prolonged mechanical ventilation and increased mortality.5–7
Risk Factors and Incidence
Most cases of ARDS in adults are associated with pneumonia with or without sepsis (60%) or with nonpulmonary sepsis (16%).10–13 Risk factors include older age and conditions that cause direct lung injury (e.g., aspiration, inhalation injury, pulmonary contusion) or indirect lung injury (e.g., drug toxicity, burns, transfusion-related acute lung injury).12,14–16 Table 1 lists signs and symptoms that suggest specific causes of ARDS.17–19

| Signs/symptoms | Possible cause |
|---|---|
| Most common | |
| Infection plus some of the following findings: temperature > 100.9°F (38.3°C) or < 96.8°F (36°C); pulse > 90 beats per minute; tachypnea; altered mental status; elevated C-reactive protein level; arterial hypotension; acute oliguria; hyperlactatemia; white blood cell count > 12,000 per mm3 (12 × 109 per L), < 4,000 mm3 (4 × 109 per L), or > 10% immature forms | Sepsis |
| Organ hypoperfusion, low mean arterial pressure, oliguria, altered mental status, elevated lactate level | Shock17 |
| Productive cough, fever, pleuritic chest pain | Pneumonia |
| Less common | |
| Bruising over the chest wall, associated injuries, history of motor vehicle crash or fall from a height | Trauma |
| Facial burns, singed eyebrows, carbonaceous sputum, history of working near organic solvents or toxic chemicals | Inhalation injury |
| Fever, rhinorrhea, cough, history of prematurity or congenital heart disease | Respiratory syncytial virus |
| History of drug abuse, especially inhalational | Drug toxicity |
| History of institutionalization, patient with developmental disabilities, or decreased Glasgow Coma Scale score | Aspiration |
| History of needing water rescue, hypothermia | Near drowning |
| Symptoms of acute lung injury and blood transfusion within the previous six hours | Transfusion-related acute lung injury |
| Special consideration | |
| Fever, cough, myalgia, fatigue, shortness of breath, diarrhea | Coronavirus disease 201918 |
According to a retrospective U.S. study, the estimated incidence of ARDS in 2014 was 193.4 cases per 100,000 people.17 A prospective Canadian study estimated the incidence to be 27.6 cases per 100,000 patient-years.16 Another study found that ARDS was responsible for 10% of ICU admissions and 23% of mechanical ventilations.12 Multiorgan failure causes most ARDS-related deaths.20,21 Mild, moderate, and severe cases of ARDS are associated with hospital mortality rates of 27% to 35%, 32% to 40%, and 46% to 60%, respectively,12,16 and hospitals with higher ARDS case volume have lower ARDS mortality.22 Risk factors for mortality include older age, worsening multiorgan dysfunction, and higher Acute Physiology and Chronic Health Evaluation (APACHE II) scores (https://www.mdcalc.com/apache-ii-score). However, age and APACHE II scores are not associated with mortality from nonpulmonary causes in patients with ARDS.20,23
Differential Diagnosis
Because the presenting symptoms of ARDS are nonspecific, physicians must consider other respiratory, cardiac, infectious, and toxic etiologies (Table 2).19 The patient history (e.g., comorbidities, exposures, medications) and physical examination focusing on the respiratory and cardiovascular systems can help narrow the differential diagnosis and determine the optimal course of treatment.
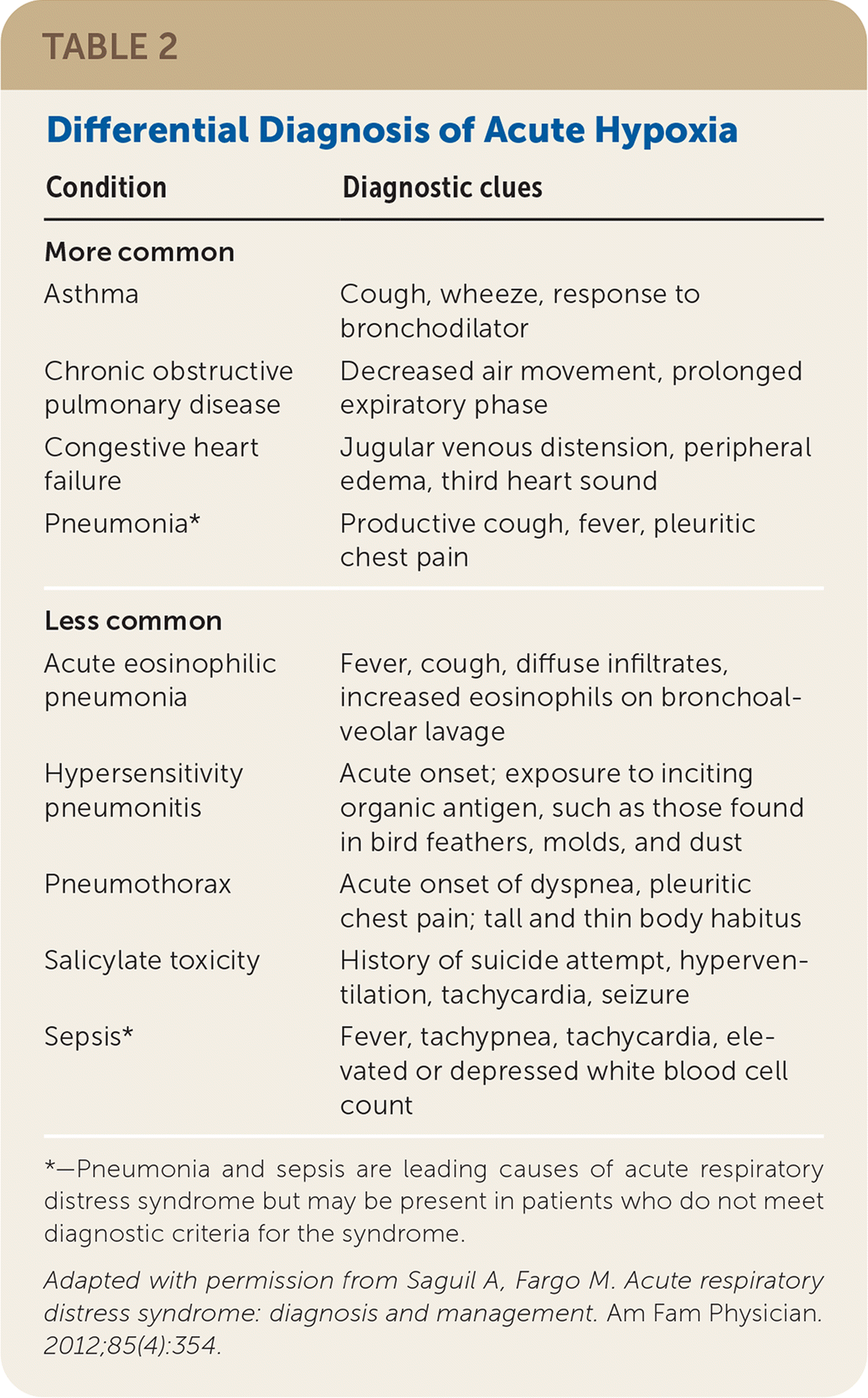
| Condition | Diagnostic clues |
|---|---|
| More common | |
| Asthma | Cough, wheeze, response to bronchodilator |
| Chronic obstructive pulmonary disease | Decreased air movement, prolonged expiratory phase |
| Congestive heart failure | Jugular venous distension, peripheral edema, third heart sound |
| Pneumonia* | Productive cough, fever, pleuritic chest pain |
| Less common | |
| Acute eosinophilic pneumonia | Fever, cough, diffuse infiltrates, increased eosinophils on bronchoalveolar lavage |
| Hypersensitivity pneumonitis | Acute onset; exposure to inciting organic antigen, such as those found in bird feathers, molds, and dust |
| Pneumothorax | Acute onset of dyspnea, pleuritic chest pain; tall and thin body habitus |
| Salicylate toxicity | History of suicide attempt, hyperventilation, tachycardia, seizure |
| Sepsis* | Fever, tachypnea, tachycardia, elevated or depressed white blood cell count |
In many cases, ARDS must be differentiated from congestive heart failure and pneumonia (Table 3).19 Unlike ARDS, congestive heart failure is characterized by fluid overload. Patients may have edema, jugular venous distension, a third heart sound, a significantly elevated brain natriuretic peptide level, and a salutary response to diuretics. Although patients with ARDS may have slightly elevated brain natriuretic peptide levels,24 they would not be expected to have these other findings.

| Distinguishing factor | ARDS | CHF | Pneumonia |
|---|---|---|---|
| Symptoms | |||
| Dyspnea | + | + | + |
| Hypoxia | + | + | + |
| Pleuritic chest pain | +/− | − | + |
| Sputum production | +/− | − | + |
| Tachypnea | + | + | + |
| Signs | |||
| Edema | − | + | − |
| Fever | +/− | − | + |
| Jugular venous distension | − | + | − |
| Rales | + | + | + |
| Third heart sound | − | + | − |
| Studies | |||
| Bilateral infiltrates | + | +/− | +/− |
| Cardiac enlargement | − | + | − |
| Elevated brain natriuretic peptide level | +/− | + | − |
| Hypoxemia | + | + | + |
| Localized infiltrate | − | − | + |
| Pao2/Fio2 ratio ≤ 300 | + | − | − |
| Pulmonary wedge pressure | + | − | + |
| ≤ 18 mm Hg | |||
| Responses | |||
| Antibiotics | − | − | + |
| Diuretics | − | + | − |
| Oxygen | − | + | + |
Pneumonia is a leading cause of ARDS, and distinguishing patients with uncomplicated pneumonia from those with ARDS can be a diagnostic challenge. In general, patients with uncomplicated pneumonia have signs of systemic and pulmonary inflammation (e.g., fever, chills, fatigue, sputum production, pleuritic chest pain, localized or multifocal infiltrates), and accompanying hypoxia should respond to oxygen administration. ARDS should be suspected if hypoxia does not resolve with supplemental oxygen.
Treatment and Support

| General measures Nutritional support Prevention of stress ulcers and venous thromboembolism Reevaluate ventilation parameters and therapeutic measures at least every 24 hours | Adjunctive measures Conservative fluid therapy Neuromuscular blockade for early severe ARDS (some guidelines also recommend for early moderate ARDS) Prone positioning for all patients with severe ARDS (some guidelines also recommend for moderate ARDS) Spontaneous breathing trials and weaning protocols Consider corticosteroids (controversial) Consider extracorporeal membrane oxygenation for severe ARDS Consider inhaled nitric oxide (controversial) Consider recruitment maneuvers (temporary elevations of applied lung pressures) |
| Ventilator parameters Choose any mode, such as volume assist Inspiratory to expiratory ratio of 1:1 to 1:3 PEEP ≥ 5 cm H2O; higher PEEP (> 12 mm Hg) preferred for moderate to severe ARDS (Fio2 ≤ 200 mm Hg)* Respiratory rate ≤ 35 breaths per minute Tidal volume of 4 to 8 mL per kg predicted body weight (start at 6 mL per kg) Arterial pH of 7.30 to 7.45 | |
| Monitoring parameters Oxygen saturation of 88% to 95% Pao2 of 55 to 80 mm Hg Plateau pressure < 30 cm H2O | |
MECHANICAL VENTILATION
Although mild cases of ARDS may respond to noninvasive ventilation, most patients require sedation, intubation, and ventilation while the underlying injury is treated. Any ventilator mode may be used,28,29 and there are multiple guidelines to inform therapy.25–27 The respiratory rate, expiratory time, PEEP, and Fio2 may be set according to protocols from the National Heart, Lung, and Blood Institute’s ARDS Network. Settings should be adjusted to maintain an oxygen saturation of 88% to 95%, an arterial pH of 7.30 to 7.45, and a plateau pressure of no more than 30 cm H2O to avoid barotrauma. Starting with low tidal volumes of 6 mL per kg of predicted body weight is superior to starting with traditional volumes of 10 to 15 mL per kg (number needed to treat to prevent death before discharge and for a patient to breathe independently = 11).29,30 Guidelines recommend initiating ventilation with low tidal volumes.25–27 However, low volumes are associated with higher partial pressures of arterial carbon dioxide (Paco2), and while some hypercapnia is permissible, Paco2 of 50 mm Hg or higher is independently associated with mortality.31 Patients should be followed closely, and adjustments should be made while balancing the preference for low tidal volumes with the risk of excessive increases in arterial carbon dioxide levels (see videos on vent basics for non-intensivists, part 1, and vent basics for non-intensivists, part 2).
Higher PEEP values (12 cm H2O or higher) are associated with decreased mortality when compared with values of 5 to 12 cm H2O (number needed to treat = 20) and should be used in patients with moderate or severe ARDS.25–27,32 Conservative fluid therapy (limiting fluid intake and considering the use of diuretics and albumin) may have some benefit but is not universally recommended.25–27
Prone positioning requires moving a patient from the traditional supine position while maintaining the integrity of the patient-ventilator circuit and all venous, arterial, urinary, and other access lines. Although the process is labor intensive and requires special training and beds, it is thought to improve ventilation-perfusion matching by recruiting more lung and allowing each inspired breath to be more uniformly distributed over a greater surface. Prone positioning is recommended for patients with severe ARDS and for those with moderate ARDS whose Pao2/Fio2 ratio is less than 150.33 When this technique is used, the position should be maintained for at least 12 to 16 hours per day.25–27
Recruitment maneuvers are temporary elevations in airway pressures intended to expand collapsed lung and increase the surface available for gas exchange. Guidelines offer mixed recommendations on recruitment maneuvers; the more recent ones recommend against them, taking into account a trial that showed increased 28-day mortality rates among patients who underwent lung recruitment.25–27,34
Inhaled nitric oxide is a pulmonary arterial vasodilator that may improve perfusion in ventilated areas and moderate the harmful inflammatory response that occurs in ARDS. It may have a role in patients with refractory disease before extracorporeal membrane oxygenation (ECMO) is considered.26,27 ECMO involves using a venoarterial or venovenous circuit to remove blood from the body, introduce oxygen and remove carbon dioxide, then return the blood to the body. Recent guidelines favor the use of ECMO in patients with severe ARDS.25–27 A randomized controlled trial found that mortality rates did not improve with the routine use of ECMO in patients with severe ARDS when compared with rescue use of ECMO in patients who do not respond to mechanical ventilation.35
Extracorporeal carbon dioxide removal also uses a venoarterial or venovenous circuit, but instead of introducing oxygen to the blood, it removes carbon dioxide. The interest in this modality centers around the hypercapnia sometimes associated with the lung-protective ventilation strategies that use low tidal volumes. Guidelines concur that more research is needed before a recommendation can be made about the use of extracorporeal carbon dioxide removal in ARDS.25–27,36,37
PHARMACOLOGIC THERAPIES
Pharmacologic options for the treatment of ARDS are limited. Surfactant therapy is not recommended for adults or children after the perinatal period.38,39 The role of corticosteroids is controversial.26 A meta-analysis of older studies, some of which enrolled patients with mild cases of ARDS and did not use lung-protective ventilation strategies, found no mortality benefit with corticosteroids.38 However, a recent multicenter randomized controlled trial in Spain found that a 10-day course of intravenous dexamethasone was associated with fewer deaths at 60 days and more ventilator-free days.40 The Surviving Sepsis Campaign guidelines recommend the use of intravenous hydrocortisone, 200 mg per day, in patients with sepsis who are hemodynamically unstable despite fluid administration and vasopressor therapy.28 Given the evolving literature, it is reasonable to consult with an intensivist about the use of corticosteroids when caring for a patient with ARDS.
Patients with ARDS should receive low-molecular-weight heparin, low-dose unfractionated heparin, or fondaparinux (Arixtra) to prevent venous thromboembolism, unless these agents are contraindicated.41 Patients with an anticipated ventilation requirement of at least 72 hours should be started on enteral nutrition. These feedings may be trophic (usually 10 to 20 mL per hour or 10 to 20 kcal per hour to maintain gut integrity and prevent mucosal atrophy) or as close to nutritional needs as possible.42,43 Stress ulcer prophylaxis should also be given.44–47 A randomized controlled trial found that intravenous pantoprazole (Protonix), 40 mg per day, decreased clinically important gastrointestinal bleeding compared with placebo.48
Ventilator Weaning
Patients with ARDS spend an average of 16 days (standard deviation = 15.8) in the ICU and a total of 26 days (standard deviation = 27.7) in the hospital.49 Patients with an anticipated ventilation requirement of more than 10 days may benefit from tracheostomy. Early tracheostomy (up to eight days after ICU admission) is associated with fewer ventilator days but not reduced long-term mortality.50,51
The American Thoracic Society and American College of Chest Physicians recommend a ventilator liberation protocol (also known as a weaning protocol) for patients who have been on mechanical ventilation for more than 24 hours.52 As the underlying illness resolves and the patient improves, spontaneous breathing trials are indicated; these protocols can reduce the duration of mechanical ventilation and weaning, and can shorten ICU stays.28,53 Table 5 outlines a set of criteria that can be used to determine whether patients can be weaned from ventilation.19

| Eligibility for starting trial Able to meet oxygen requirement with noninvasive methods Hemodynamically stable Minute ventilation ≤ 15 L Positive end-expiratory pressure ≤ 5 cm H2O |
| Parameters for ventilator weaning* Airway can be protected Hemodynamically stable No agitation Oxygen saturation ≥ 90% Respiratory frequency to tidal volume ratio ≤ 105 Respiratory rate ≤ 35 breaths per minute |
Mobilization Therapy
Patients on ventilators should be encouraged to participate in mobilization therapy consisting of range-of-motion or resistance activities, sitting, or standing. It may be necessary to reduce sedation to allow patients to participate. Mobilization therapy is associated with fewer ventilator days and a greater likelihood of being able to walk at hospital discharge.52
Post-ARDS Primary Care
After discharge from the ICU, patients with ARDS may have a lower quality of life,54 significant weakness from neuropathy or myopathy,55 persistent cognitive impairment,56 and delayed return to work.57,58 Psychiatric illness is common after ARDS: 17% to 43% of survivors report depression, 21% to 35% report posttraumatic stress disorder, and 23% to 48% report anxiety.59 Risk factors for poor physical and psychiatric outcomes include a higher APACHE II score, longer time to resolution of lung and multiorgan dysfunction, the use of systemic corticosteroids, and acquisition of illness in the ICU.60
Not all of the deleterious health effects of ARDS resolve over time. Although lung function approaches normal at five years, six-minute walking distance, physical function, and quality-of-life measures often remain decreased. Many patients report social isolation and sexual dysfunction, and more than one-half report persistent depression, anxiety, or both.61
Because the more than 100,000 people who survive ARDS each year are left with a significant burden of illness,10 it is imperative that primary care physicians initiate, coordinate, and monitor continuing services for these patients. Physicians should assess functional status at hospital follow-up and subsequent visits, ensuring that a multidimensional health care team (e.g., physical and occupational therapists, rehabilitation and home health care nurses, subspecialists) is used to promote optimal health and function. Primary care physicians should screen for mental health disorders and treat or refer patients as needed.
ARDS in Children
ARDS is less common in children and less likely to lead to death. The estimated incidence in children is 2.0 to 12.8 cases per 100,000 person-years, and the estimated mortality rate is 18% to 27%.4 Risk factors in children are similar to those in adults, including direct and indirect lung injury. Viral respiratory infection is the most common cause of ARDS in children; drowning is also a consideration.62 Treatment recommendations for children with ARDS are similar to those for adults. The recommended tidal volume is 5 to 8 mL per kg of predicted body weight (3 to 6 mL per kg in those with poor pulmonary compliance) and should be at or below the physiologic tidal volume for age and weight. Recommended PEEP values for children are 10 to 15 cm H2O for severe ARDS, and higher as needed if the plateau pressure allows. The plateau pressure should be kept at or below 28 cm H2O but may be allowed to reach 32 cm H2O for those with reduced compliance. Recruitment maneuvers are recommended in severe cases. High-frequency oscillatory ventilation can be considered for children whose plateau pressures rise above 28 cm H2O when reduced chest wall compliance is not a factor. The arterial pH may be allowed to go as low as 7.15 in conjunction with a lung-protective ventilation strategy that incorporates low tidal volumes. As with adults, children with ARDS should be evaluated daily to determine if they are eligible for ventilator weaning.39
ARDS and COVID-19
Coronavirus disease 2019 (COVID-19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARSCoV-2), is a known cause of ARDS. Clinical guidelines for ARDS care in the setting of COVID-19 have been published by the World Health Organization,63 the Centers for Disease Control and Prevention,64 the Society of Critical Care Medicine and the European Society of Intensive Care Medicine,65 and the U.S. Department of Defense (DoD).18 A full review of COVID-19 and related care is beyond the scope of this article; readers are referred to these guidelines for up-to-date information. In addition to these guidelines, free information on COVID-19 is available from American Family Physician at https://www.aafp.org/afp/COVID-19.html and Essential Evidence Plus at http://www.essentialevidenceplus.com/content/eee/904.
In a series of 24 patients with confirmed COVID-19 in Seattle-area ICUs, 18 required mechanical ventilation and met the criteria for moderate to severe ARDS.66 Initial Fio2 requirements were high, and treatment was similar to that for ARDS without COVID-19. Five patients were treated with prone positioning and seven with neuromuscular blockade. Some patients required vasopressors, but unlike typical treatment for ARDS, some received hydroxychloroquine (Plaquenil), lopinavir/ritonavir (Kaletra), or the investigational antiviral agent remdesivir. None of the patients received glucocorticoids. Of the 18 patients who required mechanical ventilation, nine died by the end of the data collection period, five remained hospitalized, and four had been discharged.
The DoD emphasizes early recognition of respiratory failure and the use of high-flow nasal cannulas instead of bilevel positive airway pressure in patients who are amenable to noninvasive management. DoD guidelines recommend the use of full personal protective equipment when intubating a patient with ARDS because of the risk of aerosolization and exposure to SARS-CoV-2.18 They recommend the ARDS Network’s protocol for PEEP and tidal volume settings, and prone positioning for patients who cannot be adequately ventilated in the supine position. The DoD guidelines do not recommend routine neuromuscular blockade or use of inhaled nitric oxide. Venovenous ECMO is an option for patients with refractory hypoxemia.
The World Health Organization likewise recommends early recognition, personal protection during intubation, and low tidal volumes.63 It encourages plateau pressures less than 30 cm H2O, prone positioning, conservative fluid management, higher PEEP values, and avoidance of routine neuromuscular blockade.
The Surviving Sepsis Campaign makes similar recommendations: the use of high-flow nasal cannulas over noninvasive positive pressure ventilation in patients with acute hypoxemic respiratory failure, low tidal volumes in those requiring mechanical ventilation, plateau pressures less than 30 cm H2O, higher (greater than 10 cm H2O) vs. lower PEEP values, a conservative fluid strategy, prone positioning and consideration of neuromuscular blockade for patients with moderate to severe ARDS, and consideration of inhaled pulmonary vasodilators and recruitment maneuvers. ECMO is recommended for patients with refractory hypoxemia.65
This article updates previous articles on this topic by the authors,19 and by Mortelliti and Manning.67
Data Sources: A PubMed search was completed using the key words acute respiratory distress syndrome and respiratory failure. The search included meta-analyses, randomized controlled trials, clinical trials, epidemiologic studies, and reviews. In addition, the websites of U.S. and international health organizations were searched for specific information on acute respiratory distress syndrome and COVID-2019. Search date: April 2020.
Editor's Note: Dr. Saguil is a contributing editor for American Family Physician.
The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Uniformed Services University of the Health Sciences or the Department of the Army.
