
Am Fam Physician. 2022;105(5):487-494
Patient information: See related handout on lung cancer, written by the authors of this article.
Published online April 1, 2022.
Author disclosure: No relevant financial relationships.
Lung cancer is the second most common cancer in men and women in the United States; however, it remains the leading cause of cancer-related death in the United States and worldwide. The most common but nonspecific symptom of lung cancer is cough. Associated symptoms, including hemoptysis or shortness of breath, or systemic symptoms, including anorexia or weight loss, greatly increase the likelihood of having lung cancer. Referral to a multidisciplinary lung cancer team, imaging, and confirmation through sputum cytology, thoracentesis, fine-needle aspiration, or mediastinoscopy are recommended. If lung cancer is confirmed, treatment options vary based on staging, histology, immunotherapy biomarker testing, and patient health status. Treatments include surgical resection, immunotherapy, chemotherapy, and/or radiotherapy. Family physicians should focus on primary prevention of lung cancer by encouraging tobacco cessation and early recognition by screening at-risk individuals and following guidelines for pulmonary nodules. As of 2021, the U.S. Preventive Services Task Force recommends annual lung cancer screening using low-dose computed tomography starting at 50 years of age in patients with a 20 pack-year history.
Lung cancer remains the leading cause of cancer-related death in the United States and worldwide; in the United States, it is the second most common cancer among men and women.1,2 The majority of lung cancers are divided into two histologic types: non–small cell lung cancer (NSCLC; 84%) and small cell lung cancer (SCLC; 13%), which helps guide treatment.3 Smoking is closely linked to 80% to 90% of lung cancer deaths, whereas radon exposure is a leading cause of nonsmoking-related lung cancer.4 Several guidelines address the management of lung cancer, with the goal of improving patient outcomes.5 In the United Kingdom, the National Institute for Health and Care Excellence has developed clinical pathways that were last updated in 2019, whereas in the United States, the most recent comprehensive lung cancer guideline from the American College of Chest Physicians was last updated in 2013, with more recent treatment recommendations from the National Comprehensive Cancer Network.2,6–8
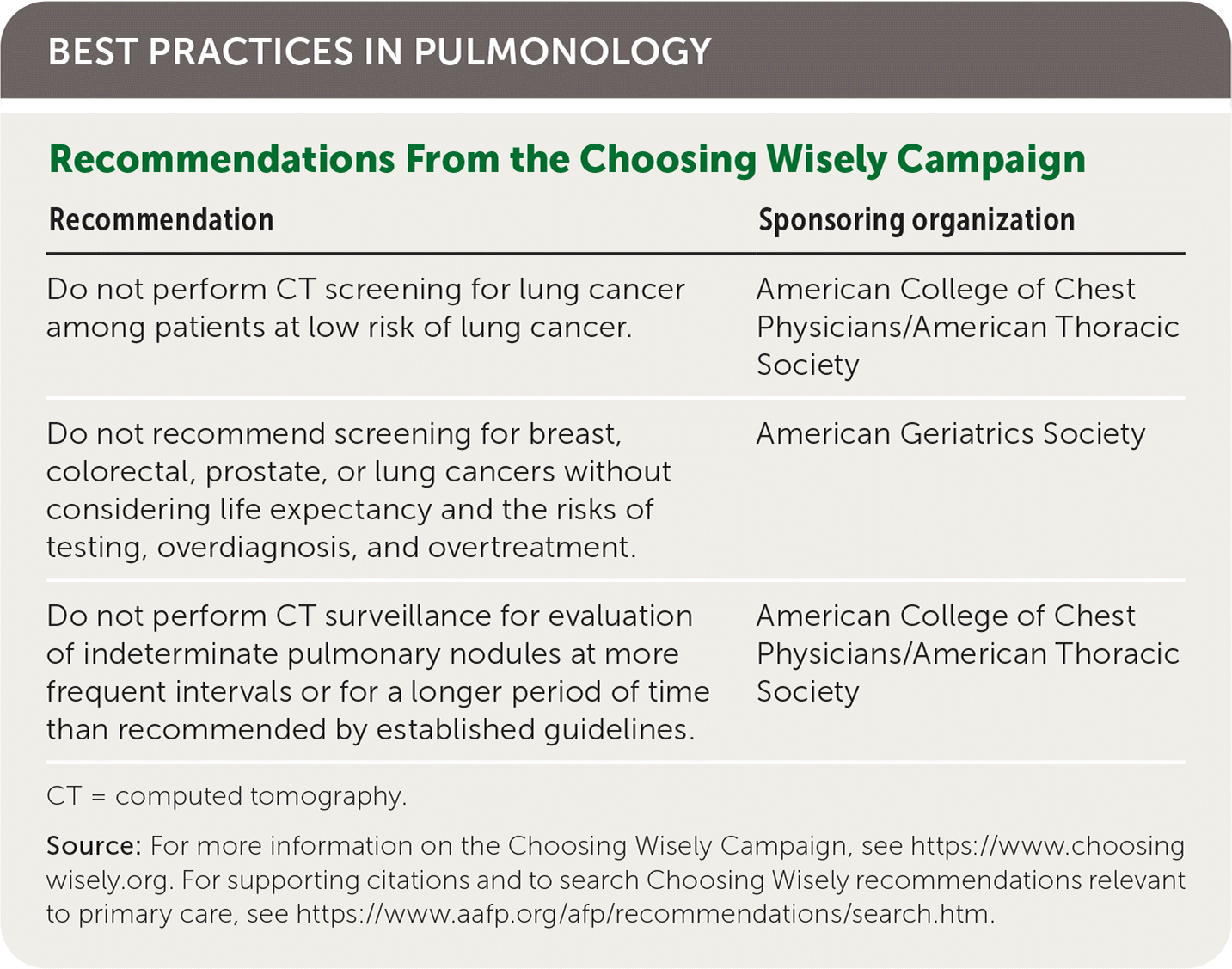
| Recommendation | Sponsoring organization |
|---|---|
| Do not perform CT screening for lung cancer among patients at low risk of lung cancer. | American College of Chest Physicians/American Thoracic Society |
| Do not recommend screening for breast, colorectal, prostate, or lung cancers without considering life expectancy and the risks of testing, overdiagnosis, and overtreatment. | American Geriatrics Society |
| Do not perform CT surveillance for evaluation of indeterminate pulmonary nodules at more frequent intervals or for a longer period of time than recommended by established guidelines. | American College of Chest Physicians/American Thoracic Society |
Clinical Presentation and Diagnosis
IN-OFFICE EVALUATION
When evaluating a patient for lung cancer, a detailed history and physical examination should be performed, including environmental and work exposures. Current smoking or history of smoking is the single most important risk factor for all types of lung cancer.9,10 Concomitant chronic lung disease or exposure to radon or asbestos may increase the risk of lung cancer.10
Patients with lung cancer typically present with symptoms,11 the most common of which is cough.9,11 Hemoptysis in combination with weight loss, loss of appetite, or shortness of breath increases the likelihood of lung cancer.11 Table 1 provides signs and symptoms of lung cancer due to local effects,12 and Table 2 and Table 3 show, respectively, advanced disease–displaying symptoms of distant metastases and paraneoplastic syndromes associated with lung cancer.12
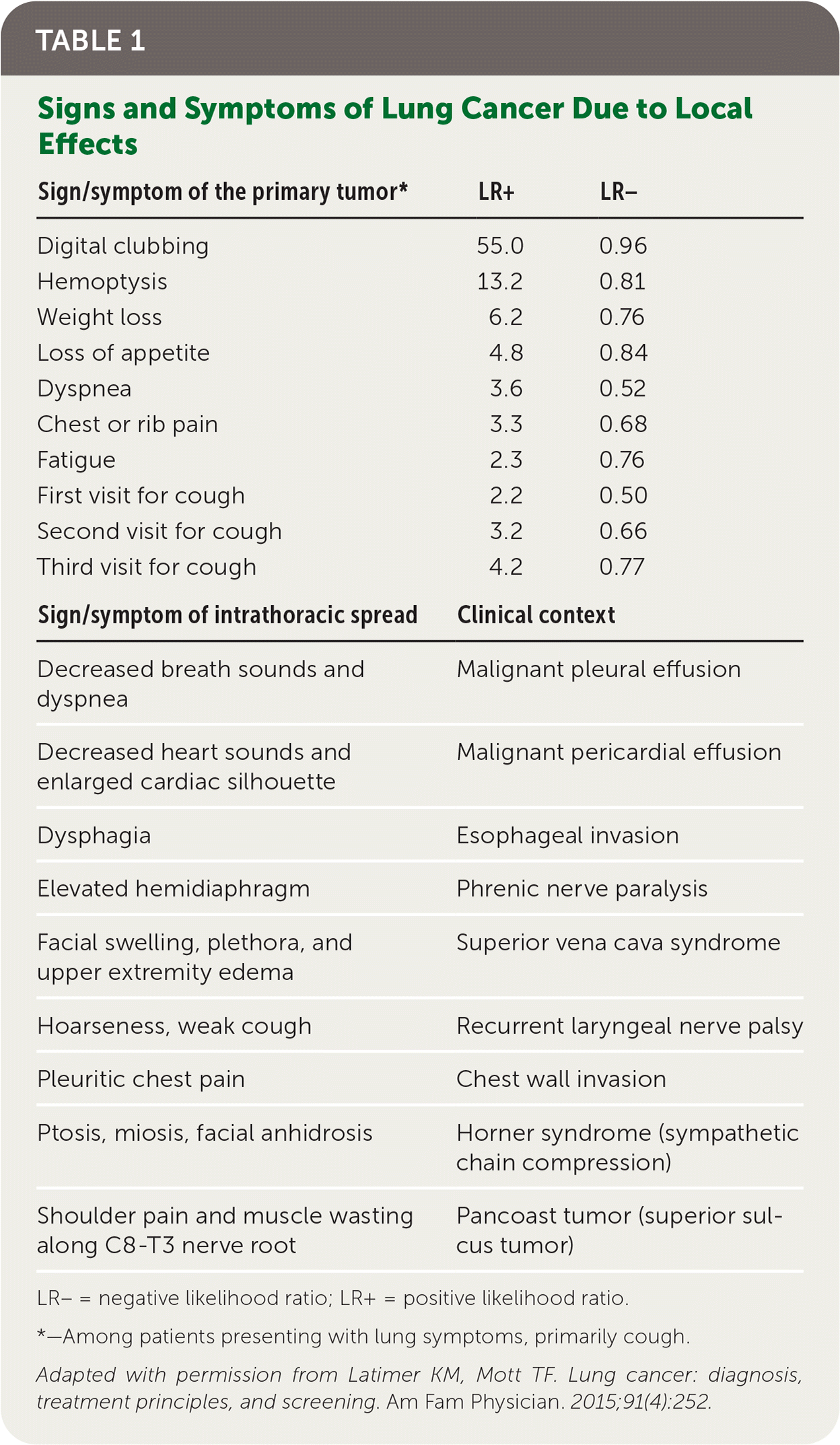
| Sign/symptom of the primary tumor* | LR+ | LR– |
|---|---|---|
| Digital clubbing | 55.0 | 0.96 |
| Hemoptysis | 13.2 | 0.81 |
| Weight loss | 6.2 | 0.76 |
| Loss of appetite | 4.8 | 0.84 |
| Dyspnea | 3.6 | 0.52 |
| Chest or rib pain | 3.3 | 0.68 |
| Fatigue | 2.3 | 0.76 |
| First visit for cough | 2.2 | 0.50 |
| Second visit for cough | 3.2 | 0.66 |
| Third visit for cough | 4.2 | 0.77 |
| Sign/symptom of intrathoracic spread | Clinical context | |
| Decreased breath sounds and dyspnea | Malignant pleural effusion | |
| Decreased heart sounds and enlarged cardiac silhouette | Malignant pericardial effusion | |
| Dysphagia | Esophageal invasion | |
| Elevated hemidiaphragm | Phrenic nerve paralysis | |
| Facial swelling, plethora, and upper extremity edema | Superior vena cava syndrome | |
| Hoarseness, weak cough | Recurrent laryngeal nerve palsy | |
| Pleuritic chest pain | Chest wall invasion | |
| Ptosis, miosis, facial anhidrosis | Horner syndrome (sympathetic chain compression) | |
| Shoulder pain and muscle wasting along C8-T3 nerve root | Pancoast tumor (superior sulcus tumor) | |
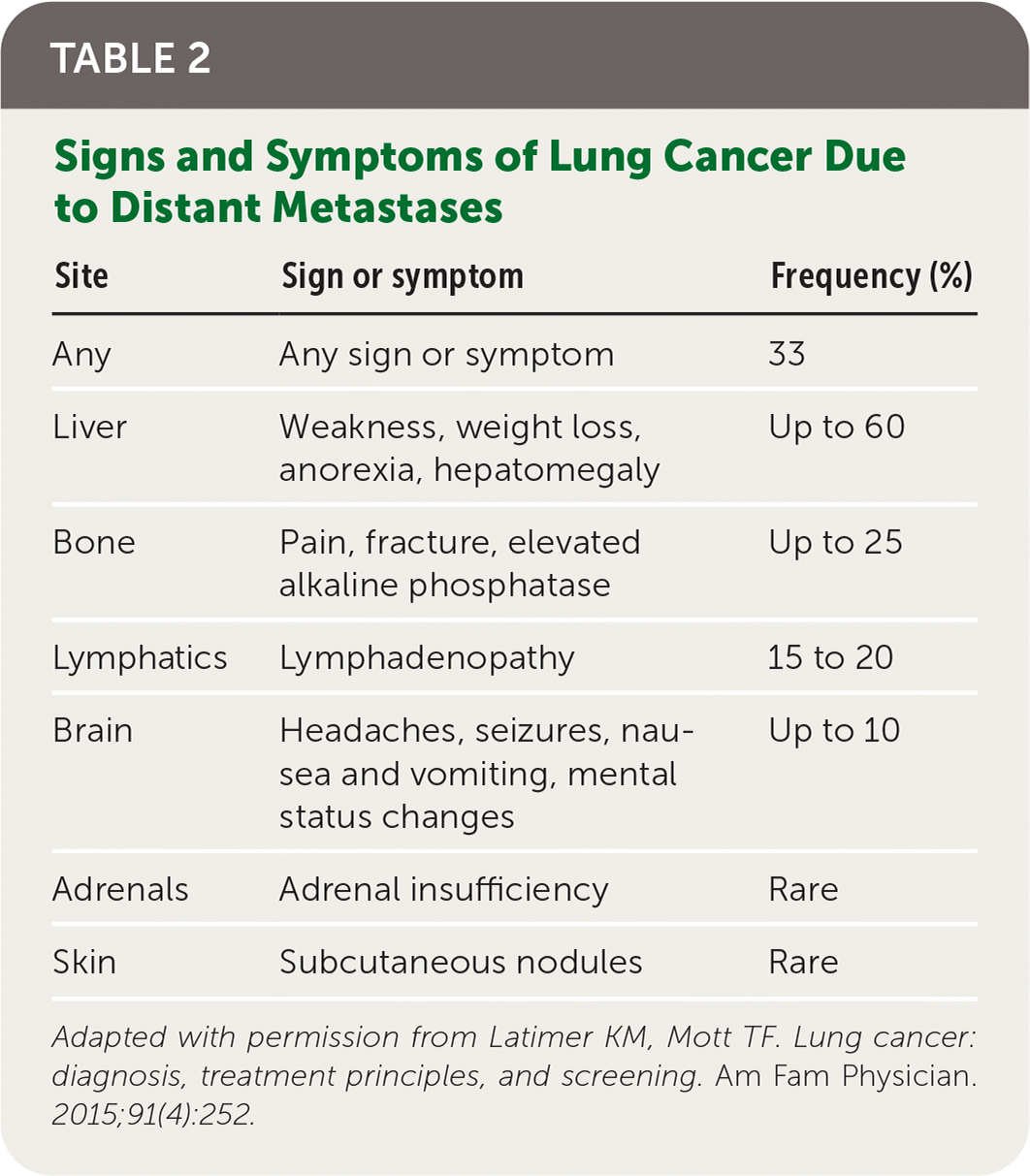
| Site | Sign or symptom | Frequency (%) |
|---|---|---|
| Any | Any sign or symptom | 33 |
| Liver | Weakness, weight loss, anorexia, hepatomegaly | Up to 60 |
| Bone | Pain, fracture, elevated alkaline phosphatase | Up to 25 |
| Lymphatics | Lymphadenopathy | 15 to 20 |
| Brain | Headaches, seizures, nausea and vomiting, mental status changes | Up to 10 |
| Adrenals | Adrenal insufficiency | Rare |
| Skin | Subcutaneous nodules | Rare |
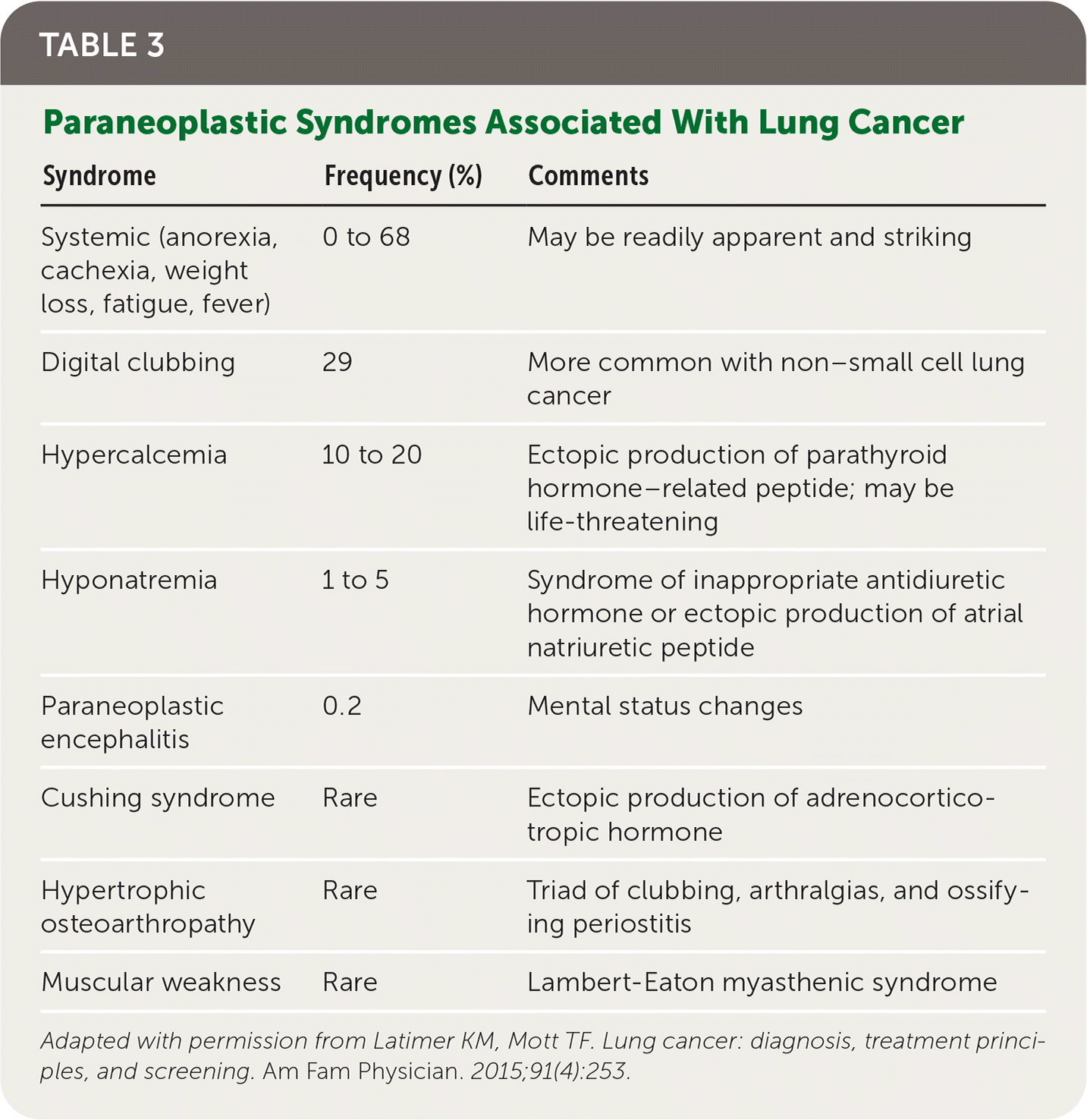
| Syndrome | Frequency (%) | Comments |
|---|---|---|
| Systemic (anorexia, cachexia, weight loss, fatigue, fever) | 0 to 68 | May be readily apparent and striking |
| Digital clubbing | 29 | More common with non–small cell lung cancer |
| Hypercalcemia | 10 to 20 | Ectopic production of parathyroid hormone–related peptide; may be life-threatening |
| Hyponatremia | 1 to 5 | Syndrome of inappropriate antidiuretic hormone or ectopic production of atrial natriuretic peptide |
| Paraneoplastic encephalitis | 0.2 | Mental status changes |
| Cushing syndrome | Rare | Ectopic production of adrenocorticotropic hormone |
| Hypertrophic osteoarthropathy | Rare | Triad of clubbing, arthralgias, and ossifying periostitis |
| Muscular weakness | Rare | Lambert-Eaton myasthenic syndrome |
The initial evaluation for patients with a suspicion for lung cancer begins with laboratory testing, including a complete blood count, serum chemistries, calcium levels, and liver function tests, with chest radiography.2,9 A normal chest radiograph alone should not be used to rule out lung cancer because just under 20% to 25% of normal chest radiographs may miss the disease.13,14 Patients who have a high level of suspicion for lung cancer based on clinical assessment or initial chest radiography findings should receive computed tomography (CT) of the chest with intravenous contrast media, ideally to include the liver and adrenals.2,15
PULMONARY NODULE FOLLOW-UP
Among patients presenting with incidental nodules found on radiographic imaging, follow-up for those older than 35 years is assessed based on features and risk categorization, as recommended by the Fleischner Society, updated in 2017 (Table 4).16,17 New studies are emerging on the use of genomic classifiers and artificial intelligence to help facilitate clinical management of incidental nodules.18,19 For patients meeting high-risk criteria and undergoing lung cancer screening, appropriate follow-up recommendations should be determined by the 2019 Lung-RADS guidelines20 (eTable A).
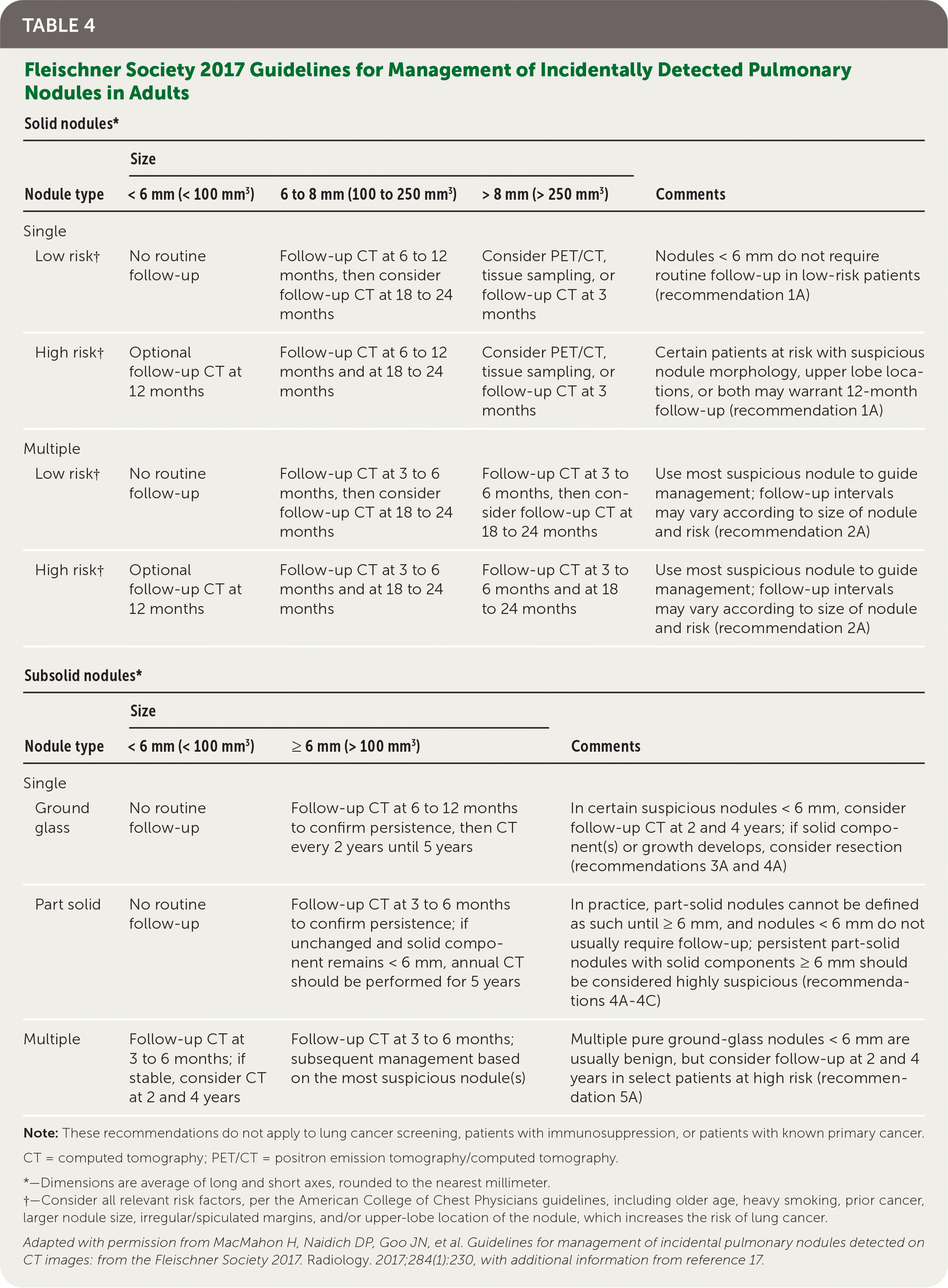
| Solid nodules* | ||||
|---|---|---|---|---|
| Nodule type | Size | Comments | ||
| < 6 mm (< 100 mm3) | 6 to 8 mm (100 to 250 mm3) | > 8 mm (> 250 mm3) | ||
| Single | ||||
| Low risk† | No routine follow-up | Follow-up CT at 6 to 12 months, then consider follow-up CT at 18 to 24 months | Consider PET/CT, tissue sampling, or follow-up CT at 3 months | Nodules < 6 mm do not require routine follow-up in low-risk patients (recommendation 1A) |
| High risk† | Optional follow-up CT at 12 months | Follow-up CT at 6 to 12 months and at 18 to 24 months | Consider PET/CT, tissue sampling, or follow-up CT at 3 months | Certain patients at risk with suspicious nodule morphology, upper lobe locations, or both may warrant 12-month follow-up (recommendation 1A) |
| Multiple | ||||
| Low risk† | No routine follow-up | Follow-up CT at 3 to 6 months, then consider follow-up CT at 18 to 24 months | Follow-up CT at 3 to 6 months, then consider follow-up CT at 18 to 24 months | Use most suspicious nodule to guide management; follow-up intervals may vary according to size of nodule and risk (recommendation 2A) |
| High risk† | Optional follow-up CT at 12 months | Follow-up CT at 3 to 6 months and at 18 to 24 months | Follow-up CT at 3 to 6 months and at 18 to 24 months | Use most suspicious nodule to guide management; follow-up intervals may vary according to size of nodule and risk (recommendation 2A) |

| Category descriptor | Lung-RADS score | Findings | Management | Risk of malignancy | Estimated population prevalence |
|---|---|---|---|---|---|
| Incomplete | 0 | Prior chest CT examination(s) being located for comparison Part or all of lungs cannot be evaluated | Additional lung cancer screening CT images and/or comparison to prior chest CT examinations is needed | NA | 1% |
| Negative: no nodules and definitely benign nodules | 1 | No lung nodules Nodule(s) with specific calcifications: complete, central, popcorn, concentric rings and fat-containing nodules | Continue annual screening with low-dose CT in 12 months | < 1% | 90% |
| Benign appearance or behavior: nodules with a very low likelihood of becoming a clinically active cancer due to size or lack of growth | 2 | Perifissural nodule(s)* < 10 mm (524 mm3) Solid nodule(s) < 6 mm total diameter (< 113 mm3) New < 4 mm (< 34 mm3) Part-solid nodule(s) < 6 mm total diameter (< 113 mm3) on baseline screening Nonsolid nodule(s) (ground-glass nodules) < 30 mm (< 14,137 mm3) or ≥ 30 mm (≥ 14,137 mm3) and unchanged or slowly growing Category 3 or 4 nodules unchanged for ≥ 3 months | Continue annual screening with low-dose CT in 12 months | < 1% | 90% |
| Probably benign finding(s): short-term follow-up suggested; includes nodules with a low likelihood of becoming a clinically active cancer | 3 | Solid nodule(s) ≥ 6 mm to < 8 mm (≥ 113 mm3 to < 268 mm3) at baseline or New 4 mm to < 6 mm (34 mm3 to < 113 mm3) Part-solid nodule(s) ≥ 6 mm total diameter (≥ 113 mm3) with solid component < 6 mm (< 113 mm3) or New < 6 mm total diameter (< 113 mm3) Nonsolid nodule(s) Ground-glass nodule ≥ 30 mm (≥ 14,137 mm3) on baseline CT or new | 6-month low-dose CT | 1% to 2% | 5% |
| Suspicious: findings for which additional diagnostic testing is recommended | 4A | Solid nodules ≥ 8 mm to < 15 mm (≥ 268 mm3 to < 1,767 mm3) at baseline or Growing < 8 mm (< 268 mm3) or New 6 mm to < 8 mm (113 mm3 to < 268 mm3) Part-solid nodules ≥ 6 mm (≥ 113 mm3) with solid component ≥ 6 mm to < 8 mm (113 mm3 to 268 mm3) or With new or growing < 4 mm (< 34 mm3) solid component Endobronchial nodule | 3-month low-dose CT; PET/CT may be used when there is a ≥ 8 mm (≥ 268 mm3) solid component | 5% to 15% | 2% |
| Very suspicious: findings for which additional diagnostic testing and/or tissue sampling is recommended | 4B | Solid nodule(s) ≥ 15 mm (≥ 1,767 mm3) or New or growing, and ≥ 8 mm (268 mm3) Part-solid nodule(s) with: A solid component ≥ 8 mm (≥ 268 mm3) or A new or growing ≥ 4 mm (≥ 34 mm3) solid component | Chest CT with or without contrast media, PET/CT, and/or tissue sampling depending on the probability of malignancy and comorbidities.† PET/CT may be used when there is a ≥ 8 mm (≥ 268 mm3) solid component. | > 15% | 2% |
| 4X | Category 3 or 4 nodules with additional features or imaging findings that increase the suspicion of malignancy | For new large nodules that develop on an annual repeat-screening CT, a 1-month low-dose CT may be recommended to address potentially infectious or inflammatory conditions | |||
| Other: clinically significant or potentially clinically significant findings (non-lung cancer) | S | Modifier: may add on to category 0–4 coding | As appropriate to the specific finding | NA | 10% |
Diagnosis Confirmation
Patients with suspected lung cancer should be referred to a pulmonologist within a multidisciplinary thoracic oncology team to help guide workup.6 Confirmation of the diagnosis should be made by one or more of the following methods, with further testing if suspicion is high and findings are negative: sputum cytology, thoracentesis of pleural fluid, bronchoscopy (often with endobronchial ultrasonography and/or electromagnetic navigation with or without fine-needle aspiration), or mediastinoscopy depending on local availability and expertise.21
STAGING
Staging of lung cancer follows the eighth edition of the American Joint Committee on Cancer's staging manual.22 Staging revisions from the seventh edition were based on analysis of a database of 94,708 cases by the International Association for the Study of Lung Cancer Staging from 1999 to 2010.23 The tumor, node, metastasis (TNM) classification describes the anatomic extent of the disease, is based on clinical and pathologic staging, and guides eventual treatment and prognosis22 (eTable B). Clinical TNM is based on history and physical examination findings, imaging, and staging procedures, and a pathologic TNM based on postsurgical histopathologic classification. The composite of these composes the TNM stage with associated prognostic stage groups I to IV22 (eTable C). TNM staging is recommended for NSCLC and SCLC for prognostic and tumor stratification purposes.22 For NSCLC, brain imaging should be performed in stage IIA patients with consideration for stage IB patients; patients with stages III to IV disease should have magnetic resonance imaging of the brain to assess for metastases even in the absence of clinical disease.7,24 Patients with any stage of SCLC should have brain imaging performed, preferably using magnetic resonance imaging.8 In patients who may undergo curative treatment, positron emission tomography CT should be performed to assess intrathoracic lymph node involvement and guide subsequent sampling.2,10
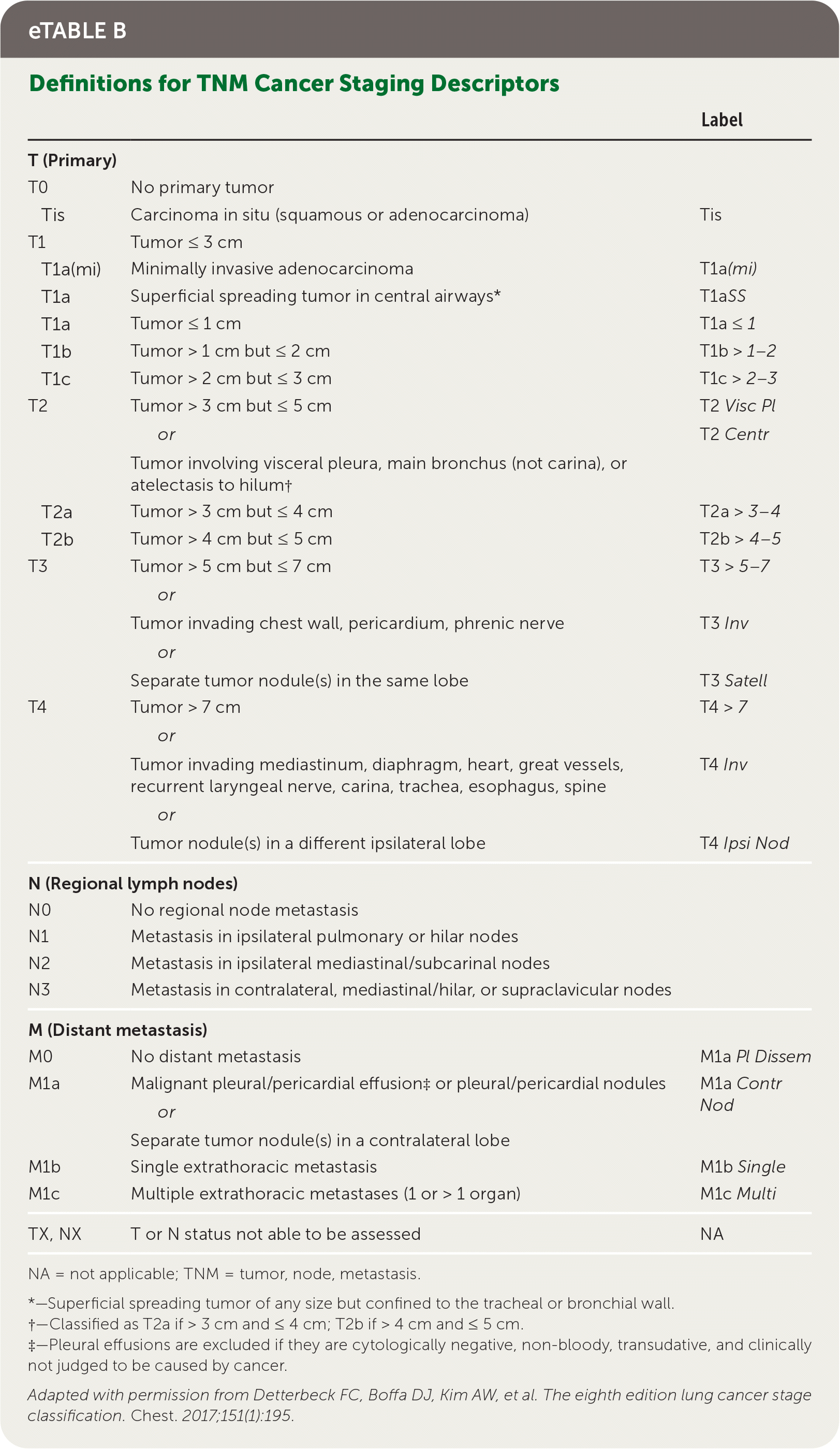
| Label | ||
|---|---|---|
| T (Primary) | ||
| T0 | No primary tumor | |
| Tis | Carcinoma in situ (squamous or adenocarcinoma) | Tis |
| T1 | Tumor ≤ 3 cm | |
| T1a(mi) | Minimally invasive adenocarcinoma | T1a (mi) |
| T1a | Superficial spreading tumor in central airways* | T1a SS |
| T1a | Tumor ≤ 1 cm | T1a ≤ 1 |
| T1b | Tumor > 1 cm but ≤ 2 cm | T1b > 1–2 |
| T1c | Tumor > 2 cm but ≤ 3 cm | T1c > 2–3 |
| T2 | Tumor > 3 cm but ≤ 5 cm or Tumor involving visceral pleura, main bronchus (not carina), or atelectasis to hilum† | T2 Visc Pl T2 Centr |
| T2a | Tumor > 3 cm but ≤ 4 cm | T2a > 3–4 |
| T2b | Tumor > 4 cm but ≤ 5 cm | T2b > 4–5 |
| T3 | Tumor > 5 cm but ≤ 7 cm or Tumor invading chest wall, pericardium, phrenic nerve or Separate tumor nodule(s) in the same lobe | T3 > 5–7 T3 Inv T3 Satell |
| T4 | Tumor > 7 cm or Tumor invading mediastinum, diaphragm, heart, great vessels, recurrent laryngeal nerve, carina, trachea, esophagus, spine or Tumor nodule(s) in a different ipsilateral lobe | T4 > 7 T4 Inv T4 Ipsi Nod |
| N (Regional lymph nodes) | ||
| N0 | No regional node metastasis | |
| N1 | Metastasis in ipsilateral pulmonary or hilar nodes | |
| N2 | Metastasis in ipsilateral mediastinal/subcarinal nodes | |
| N3 | Metastasis in contralateral, mediastinal/hilar, or supraclavicular nodes | |
| M (Distant metastasis) | ||
| M0 | No distant metastasis | M1a Pl Dissem |
| M1a | Malignant pleural/pericardial effusion‡ or pleural/pericardial nodules or Separate tumor nodule(s) in a contralateral lobe | M1a Contr Nod |
| M1b | Single extrathoracic metastasis | M1b Single |
| M1c | Multiple extrathoracic metastases (1 or > 1 organ) | M1c Multi |
| TX, NX | T or N status not able to be assessed | NA |
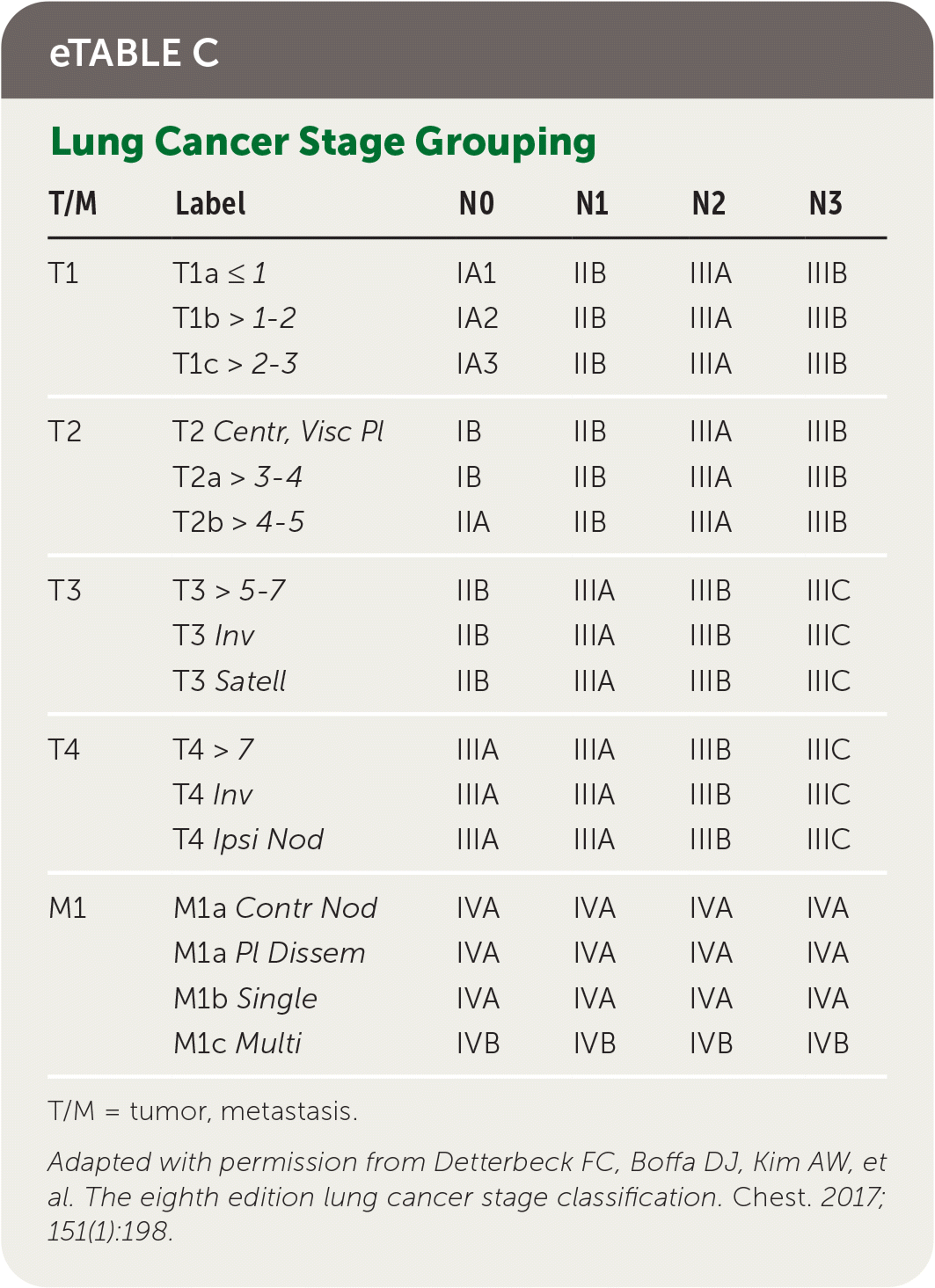
| T/M | Label | N0 | N1 | N2 | N3 |
|---|---|---|---|---|---|
| T1 | T1a ≤ 1 | IA1 | IIB | IIIA | IIIB |
| T1b > 1–2 | IA2 | IIB | IIIA | IIIB | |
| T1c > 2–3 | IA3 | IIB | IIIA | IIIB | |
| T2 | T2 Centr, Visc Pl | IB | IIB | IIIA | IIIB |
| T2a > 3–4 | IB | IIB | IIIA | IIIB | |
| T2b > 4–5 | IIA | IIB | IIIA | IIIB | |
| T3 | T3 > 5–7 | IIB | IIIA | IIIB | IIIC |
| T3 Inv | IIB | IIIA | IIIB | IIIC | |
| T3 Satell | IIB | IIIA | IIIB | IIIC | |
| T4 | T4 > 7 | IIIA | IIIA | IIIB | IIIC |
| T4 Inv | IIIA | IIIA | IIIB | IIIC | |
| T4 Ipsi Nod | IIIA | IIIA | IIIB | IIIC | |
| M1 | M1a Contr Nod | IVA | IVA | IVA | IVA |
| M1a Pl Dissem | IVA | IVA | IVA | IVA | |
| M1b Single | IVA | IVA | IVA | IVA | |
| M1c Multi | IVB | IVB | IVB | IVB |
Treatment
NON–SMALL CELL LUNG CANCER
The treatment of NSCLC varies based on staging, nonsquamous (usually adenocarcinoma) vs. squamous histology, and genetic and immunotherapy biomarker testing. Treatment options presented here provide an overview; however, specific regimens will vary based on the availability of treatment options and clinical experience of the multidisciplinary treatment team. Patients with advanced disease should be offered early palliative care.7
Patients with stages I to II NSCLC are usually offered a combination of three treatments: surgery, which can include complete resection of the tumor (usually stages I and II), and mediastinal lymph node dissection or lymph node sampling; radiotherapy; and adjuvant platinum-based chemotherapy.25 Select patients who have stage III NSCLC but do not have disease progression after chemotherapy may benefit from immunotherapy.7,26 Video-assisted thoracic surgery has lower mortality and hospital length of stay compared with open thoracotomy.27 Nonsurgical candidates can be offered radiotherapy and platinum-based chemotherapy.28 For patients with stage IV disease, palliative care and immunotherapy with or without platinum-based chemotherapy are recommended.7 In patients with fewer than three brain metastases, stereotactic radiotherapy or surgery with stereotactic radiotherapy is recommended.29 With more than three brain metastases, whole brain radiation is recommended, although it may not improve neurocognitive symptoms or overall survival.28,29 Radiotherapy and bisphosphonates are recommended for bone metastases to reduce pain and risk of skeletal fractures.28,29
All patients who have NSCLC with nonsquamous NSCLC, mixed histology, or small-volume biopsies should be offered genetic and immunotherapy testing (e.g., broad-based, next-generation sequencing).7 Common driver mutations, preferred treatment options, and common adverse effects are listed in eTable D. Genetic testing can predict overall prognosis and responsiveness to targeted therapies; however, U.S. Food and Drug Administration–approved therapies depend on histologic subtype, disease progression, and timing with first-line systemic chemo-therapy.7 Standard first-line therapy for advanced NSCLC is immunotherapy with or without chemotherapy, based on PD-L1 (programmed death-ligand 1) status of expression on tumor cells.7
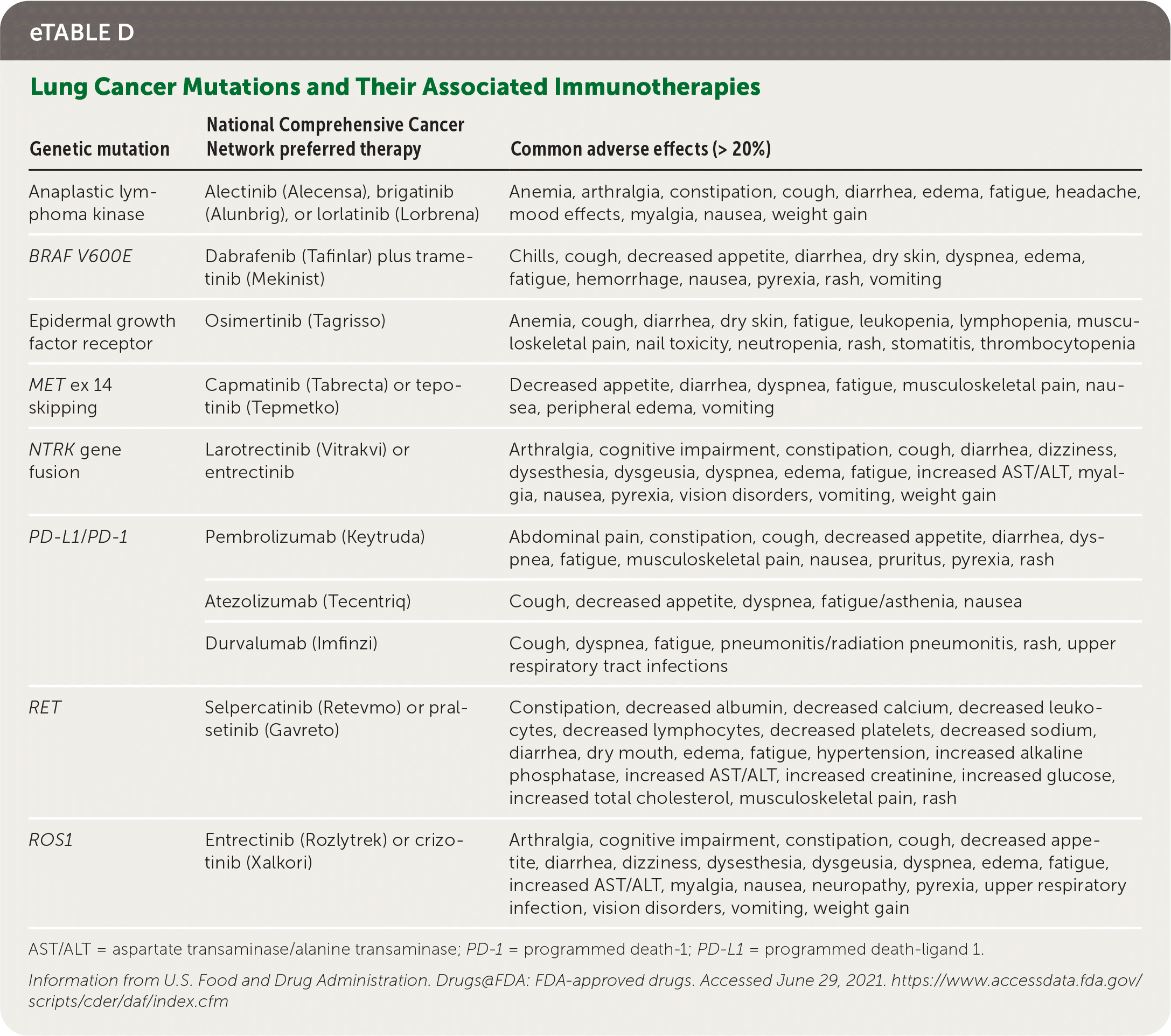
| Genetic mutation | National Comprehensive Cancer Network preferred therapy | Common adverse effects (> 20%) |
|---|---|---|
| Anaplastic lymphoma kinase | Alectinib (Alecensa), brigatinib (Alunbrig), or lorlatinib (Lorbrena) | Anemia, arthralgia, constipation, cough, diarrhea, edema, fatigue, headache, mood effects, myalgia, nausea, weight gain |
| BRAF V600E | Dabrafenib (Tafinlar) plus trametinib (Mekinist) | Chills, cough, decreased appetite, diarrhea, dry skin, dyspnea, edema, fatigue, hemorrhage, nausea, pyrexia, rash, vomiting |
| Epidermal growth factor receptor | Osimertinib (Tagrisso) | Anemia, cough, diarrhea, dry skin, fatigue, leukopenia, lymphopenia, musculoskeletal pain, nail toxicity, neutropenia, rash, stomatitis, thrombocytopenia |
| MET ex 14 skipping | Capmatinib (Tabrecta) or tepotinib (Tepmetko) | Decreased appetite, diarrhea, dyspnea, fatigue, musculoskeletal pain, nausea, peripheral edema, vomiting |
| NTRK gene fusion | Larotrectinib (Vitrakvi) or entrectinib | Arthralgia, cognitive impairment, constipation, cough, diarrhea, dizziness, dysesthesia, dysgeusia, dyspnea, edema, fatigue, increased AST/ALT, myalgia, nausea, pyrexia, vision disorders, vomiting, weight gain |
| PD-L1/ PD-1 | Pembrolizumab (Keytruda) | Abdominal pain, constipation, cough, decreased appetite, diarrhea, dyspnea, fatigue, musculoskeletal pain, nausea, pruritus, pyrexia, rash |
| Atezolizumab (Tecentriq) | Cough, decreased appetite, dyspnea, fatigue/asthenia, nausea | |
| Durvalumab (Imfinzi) | Cough, dyspnea, fatigue, pneumonitis/radiation pneumonitis, rash, upper respiratory tract infections | |
| RET | Selpercatinib (Retevmo) or pralsetinib (Gavreto) | Constipation, decreased albumin, decreased calcium, decreased leukocytes, decreased lymphocytes, decreased platelets, decreased sodium, diarrhea, dry mouth, edema, fatigue, hypertension, increased alkaline phosphatase, increased AST/ALT, increased creatinine, increased glucose, increased total cholesterol, musculoskeletal pain, rash |
| ROS1 | Entrectinib (Rozlytrek) or crizotinib (Xalkori) | Arthralgia, cognitive impairment, constipation, cough, decreased appetite, diarrhea, dizziness, dysesthesia, dysgeusia, dyspnea, edema, fatigue, increased AST/ALT, myalgia, nausea, neuropathy, pyrexia, upper respiratory infection, vision disorders, vomiting, weight gain |
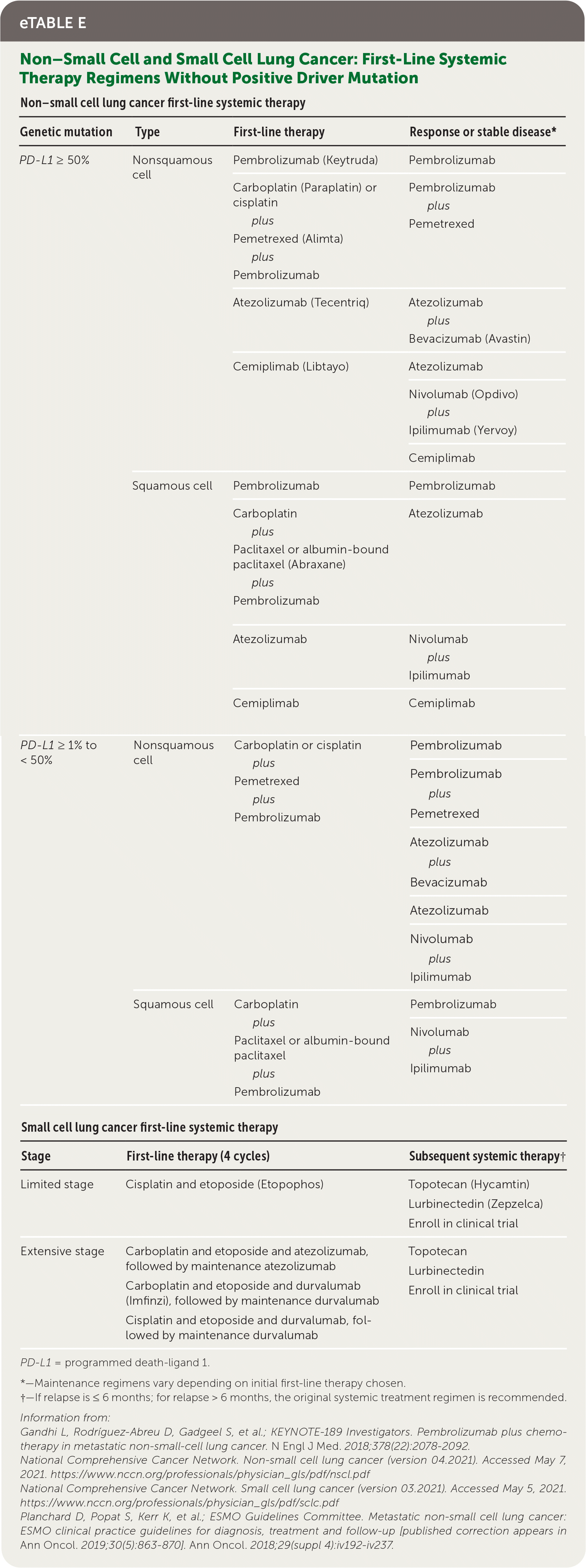
| Non–small cell lung cancer first-line systemic therapy | |||
|---|---|---|---|
| Genetic mutation | Type | First-line therapy | Response or stable disease* |
| PD-L1 ≥ 50% | Nonsquamous cell | Pembrolizumab (Keytruda) | Pembrolizumab |
| Carboplatin (Paraplatin) or cisplatin plus Pemetrexed (Alimta) plus Pembrolizumab | Pembrolizumab plus Pemetrexed | ||
| Atezolizumab (Tecentriq) | Atezolizumab plus Bevacizumab (Avastin) | ||
| Cemiplimab (Libtayo) | Atezolizumab | ||
| Nivolumab (Opdivo) plus Ipilimumab (Yervoy) | |||
| Cemiplimab | |||
| Squamous cell | Pembrolizumab | Pembrolizumab | |
| Carboplatin plus Paclitaxel or albumin-bound paclitaxel (Abraxane) plus Pembrolizumab | Atezolizumab | ||
| Atezolizumab | Nivolumab plus Ipilimumab | ||
| Cemiplimab | Cemiplimab | ||
| PD-L1 ≥ 1% to < 50% | Nonsquamous cell | Carboplatin or cisplatin plus Pemetrexed plus Pembrolizumab | Pembrolizumab |
| Pembrolizumab plus Pemetrexed | |||
| Atezolizumab plus Bevacizumab | |||
| Atezolizumab | |||
| Nivolumab plus Ipilimumab | |||
| Squamous cell | Carboplatin plus Paclitaxel or albumin-bound paclitaxel plus Pembrolizumab | Pembrolizumab | |
| Nivolumab plus Ipilimumab | |||
| Small cell lung cancer first-line systemic therapy | |||
| Stage | First-line therapy (4 cycles) | Subsequent systemic therapy† | |
| Limited stage | Cisplatin and etoposide (Etopophos) | Topotecan (Hycamtin) Lurbinectedin (Zepzelca) Enroll in clinical trial | |
| Extensive stage | Carboplatin and etoposide and atezolizumab, followed by maintenance atezolizumab Carboplatin and etoposide and durvalumab (Imfinzi), followed by maintenance durvalumab Cisplatin and etoposide and durvalumab, followed by maintenance durvalumab | - | Topotecan Lurbinectedin Enroll in clinical trial |
In 2017, five-year survival for localized NSCLC was 59%, with only 5.8% for five-year survival in patients with distant metastases; however, there have been reductions in mortality since 2013 likely due to a decrease in incidence and advancements in therapies, as described previously 31 (Table 522).
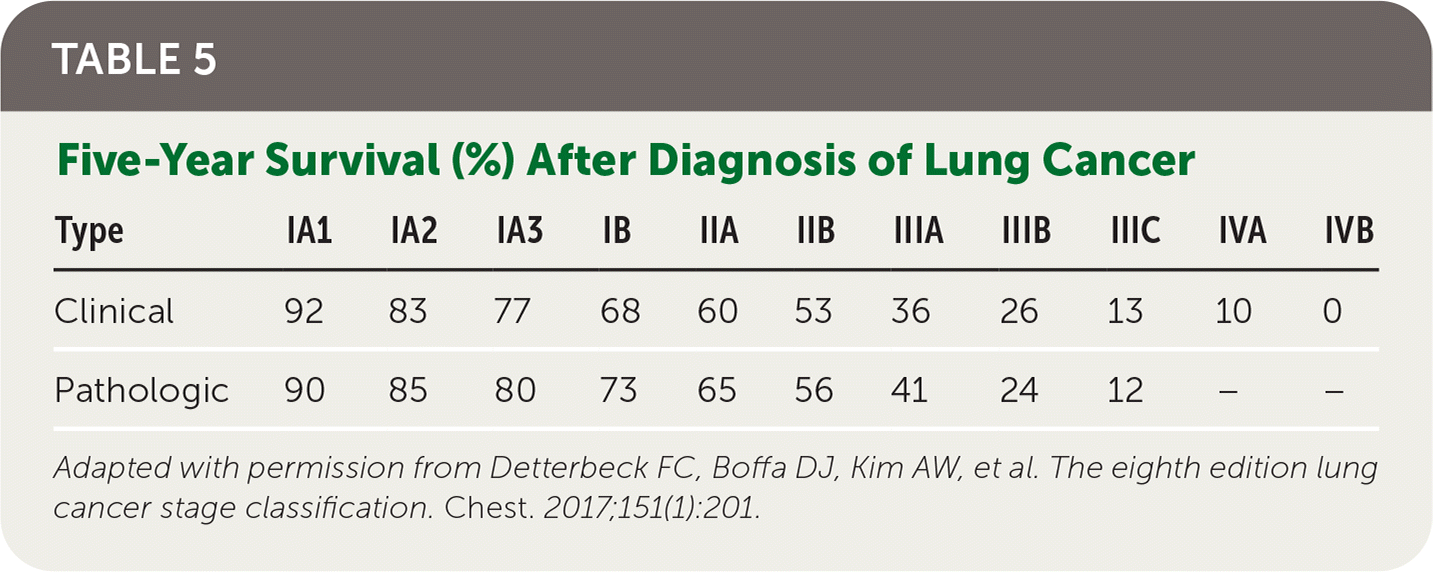
| Type | IA1 | IA2 | IA3 | IB | IIA | IIB | IIIA | IIIB | IIIC | IVA | IVB |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical | 92 | 83 | 77 | 68 | 60 | 53 | 36 | 26 | 13 | 10 | 0 |
| Pathologic | 90 | 85 | 80 | 73 | 65 | 56 | 41 | 24 | 12 | – | – |
SMALL CELL LUNG CANCER
For patients with limited-stage SCLC, the standard of care is etoposide (Etopophos) plus cisplatin chemotherapy and concurrent thoracic radiotherapy, with surgical resection offered in select patients.8,32 Patients with significant comorbidities, including chronic kidney disease, may be offered an alternative carboplatin (Paraplatin)–based chemotherapy regimen with similar effectiveness.32 For patients with extensive-stage SCLC, four to six cycles of one of several combination chemotherapy/immunotherapy regimens should be offered with maintenance immunotherapy.8 Consolidative thoracic radiation may be considered for select patients with residual intrathoracic disease who have responded to systemic chemotherapy.8 In patients with limited-stage SCLC, prophylactic cranial irradiation for brain metastases reduces mortality.33 Localized palliative radiation for nonpulmonary sites, including whole brain radiotherapy for brain metastases, should be offered.28 Patients with relapse after initial therapy have overall poor prognosis; however, several second-line systemic therapy options are available. 8,34 Prognosis remains poor, with only 20% to 25% five-year survival for limited-stage SCLC and less than 10% two-year survival for extensive-stage SCLC35 (Table 522).
Screening
As of 2021, the U.S. Preventive Services Task Force (USPSTF) has recommended annual low-dose CT screening in adults 50 to 80 years of age who have a 20 pack-year smoking history and currently smoke or have quit smoking within the past 15 years.36 This replaces the 2013 recommendation of annual CT screenings for patients 55 to 80 years of age with at least a 30 pack-year history.37 The criteria for discontinuing screening are unchanged, including patients who have quit smoking for more than 15 years, have limited life expectancies (less than 10 years), or are not willing to undergo curative lung surgery.36
The updated recommendation is based on two major randomized controlled trials, the National Lung Screening Trial and the Dutch–Belgian lung-cancer screening trial (Nederlands–Leuvens Longkanker Screenings Onderzoek).38,39 Both of these trials found reductions in lung cancer mortality, with a number needed to screen to prevent one lung cancer death of 323 over 6.5 years of follow-up and 130 over 10 years of follow-up, respectively.38–40 Through systematic review of these trials and modeling studies from the Cancer Intervention and Surveillance Modeling Network, the USPSTF updated its criteria for screening.36 Earlier screening recommendations are based on studies that suggest this may help address screening disparities for certain populations, including women and Black and Hispanic people.41,42 Compared with the previous USPSTF 2013 guideline, Cancer Intervention and Surveillance Modeling Network data suggest that earlier screenings would be associated with an increase in the reduction of lung cancer mortality, from a 9.8% reduction to 12.1% to 14.4%, and life-years gained, from 4,882 life-years to 6,018 to 7,596 per 100,000.37,43 The American Academy of Family Physicians supports the USPSTF's grade B recommendation of lung cancer screening in adults at increased risk; however, the harms of annual CT screenings are still not well documented, and further research is needed.44 Research gaps include evaluating potential harms associated with increased radiation exposure, identifying better technology to differentiate benign and malignant lung nodules to avoid overdiagnosis, and addressing the cost and availability of increased screening in economically disadvantaged populations.44
Smoking Cessation and Counseling
Smoking cessation reduces morbidity and mortality in patients with lung cancer; however, no randomized controlled trials have compared specific cessation interventions in this population.29,45 Exercise training may improve exercise capacity and quality of life.46 Nursing interventions can help patients with dyspnea, and a range of psychological interventions may improve coping skills and quality of life.47
Although all actively smoking patients should be offered cessation support, lung cancer screening for eligible patients coupled with cessation support may be associated with higher quitting rates.48 This combination is believed to serve as a teachable moment during a time when patients are the most receptive to quitting advice. Cessation assistance in combination with CT screening has been associated with a reduction in lung cancer–specific mortality and the potential to improve the cost-effectiveness ratio of screening.49,50 Patients who quit smoking have been shown to reduce their risk of lung cancer by 39.1% after five years.51 Patients should also be counseled that quitting smoking will reduce their risk of all second cancers by 3.5 times.52
This article updates previous articles on this topic by Latimer and Mott12 and Collins, et al.53
Data Sources: A PubMed search was completed in Clinical Queries using the key terms lung cancer, diagnosis, treatment, and screening. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. The Agency for Healthcare Research and Quality Effective Healthcare Reports, the U.S. Preventive Services Task Force, the Cochrane Database of Systematic Reviews, DynaMed, Essential Evidence Plus, the National Institute for Health and Care Excellence, and the National Comprehensive Cancer Network were also searched. Search dates: April and May 2021, and January 28, 2022.
The authors thank Hamid Mirshahidi, MD, associate professor of medicine, hematology and oncology, and Laren Tan, MD, associate professor of medicine, pulmonary and critical care, Loma Linda University School of Medicine, for thoughtful advice and review of the manuscript.
