
Am Fam Physician. 2016;93(6):468-474
Patient information: See related handout on endometrial (uterine) cancer, written by the authors of this article.
Author disclosure: No relevant financial affiliations.
Endometrial cancer is the most common gynecologic malignancy. It is the fourth most common cancer in women in the United States after breast, lung, and colorectal cancers. Risk factors are related to excessive unopposed exposure of the endometrium to estrogen, including unopposed estrogen therapy, early menarche, late menopause, tamoxifen therapy, nulliparity, infertility or failure to ovulate, and polycystic ovary syndrome. Additional risk factors are increasing age, obesity, hypertension, diabetes mellitus, and hereditary nonpolyposis colorectal cancer. The most common presentation for endometrial cancer is postmenopausal bleeding. The American Cancer Society recommends that all women older than 65 years be informed of the risks and symptoms of endometrial cancer and advised to seek evaluation if symptoms occur. There is no evidence to support endometrial cancer screening in asymptomatic women. Evaluation of a patient with suspected disease should include a pregnancy test in women of childbearing age, complete blood count, and prothrombin time and partial thromboplastin time if bleeding is heavy. Most guidelines recommend either transvaginal ultrasonography or endometrial biopsy as the initial study. The mainstay of treatment for endometrial cancer is total hysterectomy with bilateral salpingo-oophorectomy. Radiation and chemotherapy can also play a role in treatment. Low- to medium-risk endometrial hyperplasia can be treated with nonsurgical options. Survival is generally defined by the stage of the disease and histology, with most patients at stage I and II having a favorable prognosis. Controlling risk factors such as obesity, diabetes, and hypertension could play a role in the prevention of endometrial cancer.
Endometrial cancer is the most common gynecologic malignancy. It is the fourth most common cancer in women after breast, lung, and colorectal cancers. Projections from the American Cancer Society (ACS) for 2015 estimated 54,870 new cases of endometrial cancer and 10,170 deaths from the disease.1 The death rate for endometrial cancer has increased more than 100% during the past 20 years, rising by 8% since 2008. The mean age of patients at the time of diagnosis is 63 years, with 90% of cases occurring in women older than 50 years. Only 20% of patients with endometrial cancer receive a diagnosis before menopause.2
WHAT IS NEW ON THIS TOPIC: ENDOMETRIAL CANCER
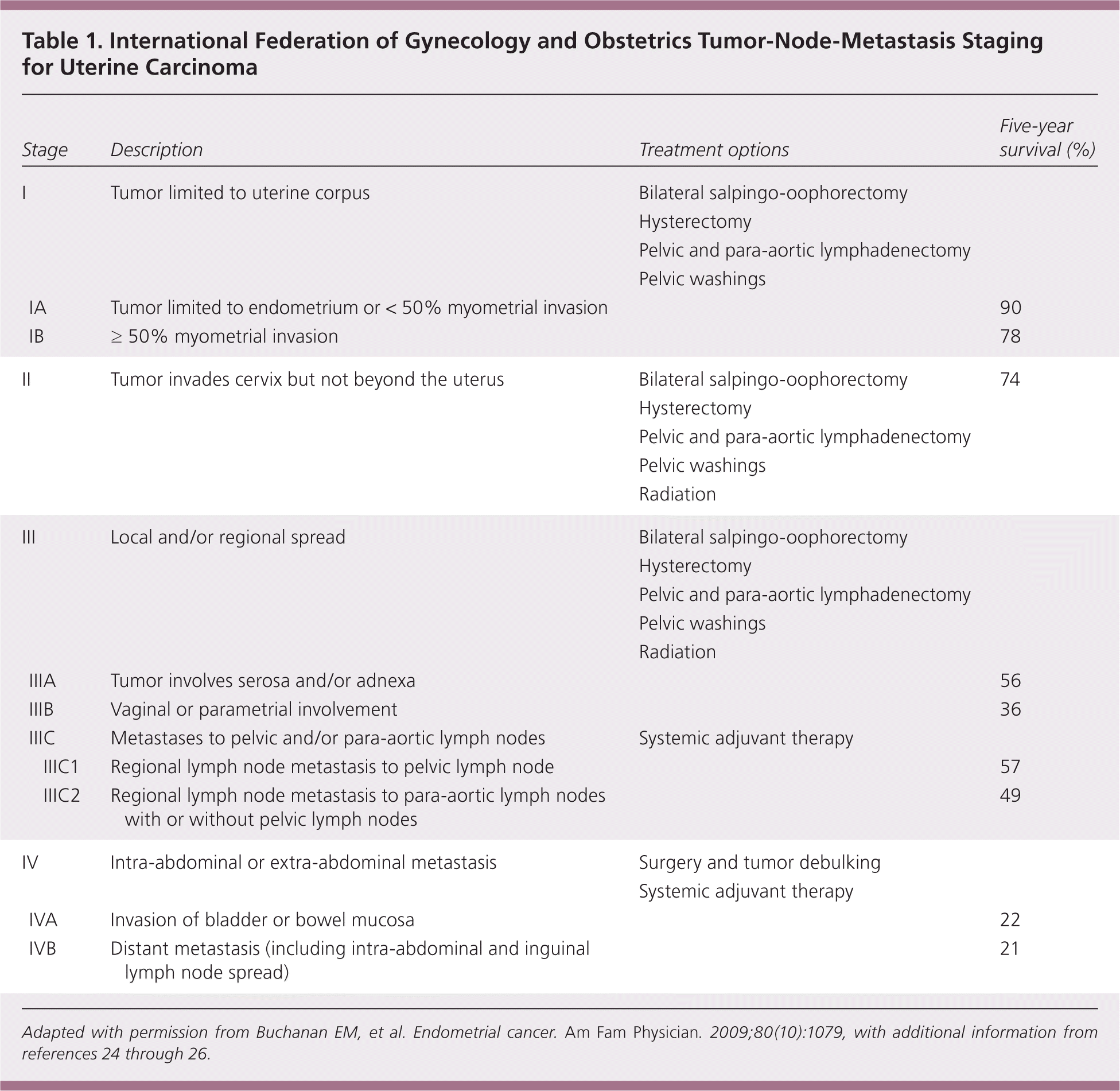
The 2009 update of the International Federation of Gynecology and Obstetrics tumor-node-metastasis staging system for endometrial cancer better predicts disease prognosis compared with the previous system (Table 1).

| Recommendation | Sponsoring organization |
|---|---|
| Do not perform Papanicolaou tests for surveillance of women with a history of endometrial cancer. | Society of Gynecologic Oncology |
| Clinical recommendation | Evidence rating | References | Comments |
|---|---|---|---|
| Women older than 65 years should be informed of the risks and symptoms of endometrial cancer and advised to seek evaluation if symptoms occur. | C | 4 | Recommendation based on consensus guidelines |
| Women with abnormal uterine bleeding should be evaluated for endometrial cancer if they are older than 45 years or if they have a history of unopposed estrogen exposure. | C | 2, 3, 19 | Recommendation based on consensus guidelines |
| In postmenopausal women, the endometrial thickness on transvaginal ultrasonography should be less than 4 to 5 mm. With thickness above this level, biopsy should be considered to rule out endometrial hyperplasia or cancer. | C | 17, 18 | Recommendation based on consensus guidelines |
Histopathology
Endometrial cancer is generally classified into two types.2 Type I is the most common form, representing more than 70% of cases. Type I tumors are associated with unopposed estrogen stimulation and are known as endometrioid adenocarcinoma.3 These tumors are generally low grade. Type II tumors are more likely to be high grade and of papillary serous or clear cell histologic type. They carry a poor prognosis and have a high risk of relapse and metastasis. Type II accounts for only 10% of endometrial cancers, but it is associated with 40% of related deaths.2,3 Familial tumors are commonly found in association with Lynch syndrome (hereditary nonpolyposis colorectal cancer). Genetic disease represents 10% of cases of endometrial cancer.3
Endometrial hyperplasia represents a precursor lesion to endometrial cancer. Hyperplasia carries a 1% to 3% risk of progression to cancer. Atypical hyperplasia is associated with greater cancer risk than simple or complex hyperplasia; 30% to 40% of patients with atypical hyperplasia have concomitant adenocarcinoma.2
Risk Factors
Risk factors for type I endometrial cancer are related to unopposed exposure of the endometrium to estrogen, including unopposed estrogen therapy, early menarche, late menopause, tamoxifen therapy, nulliparity, infertility or failure to ovulate, and polycystic ovary syndrome. Other risk factors not involving unopposed estrogen include family history of endometrial cancer, age older than 50 years, hypertension, diabetes mellitus, obesity, thyroid disease, and Lynch syndrome.2–6 Although they are less common overall, type II tumors are found predominantly in black women older than 50 years.2
Screening and Prevention
The ACS recommends that all women older than 65 years be informed of the risks and symptoms of endometrial cancer and advised to seek evaluation if symptoms occur. There is no evidence to support the screening of asymptomatic women, with the exception of those who have or are at increased risk of Lynch syndrome.4 Although the recommendation is controversial, these patients should be screened annually with an endometrial biopsy starting at age 35 because of a 22% to 50% lifetime risk of endometrial cancer.4,12
There are no recommendations on screening for endometrial cancer in patients who are taking tamoxifen; however, those who present with abnormal uterine bleeding should be considered for diagnostic workup. Use of the levonorgestrel-releasing intrauterine system (Mirena) has not been proven to protect against endometrial hyperplasia or cancer in patients who take tamoxifen.15
Management of risk factors such as obesity, diabetes, and hypertension could play a role in the prevention of endometrial cancer. For women on hormone therapy, the addition of progesterone has been shown to decrease the risk of endometrial cancer.16
History
Vaginal bleeding is the most common clinical presentation of endometrial cancer in postmenopausal women.2,17,18 Approximately 75% of postmenopausal women who are diagnosed with endometrial cancer are diagnosed at an early stage, which improves the chances of successful treatment.2 However, only 10% to 20% of postmenopausal women who are evaluated for uterine bleeding are diagnosed with endometrial cancer because the most common cause of postmenopausal bleeding is endometrial atrophy.17,18 All postmenopausal bleeding should be investigated, especially if risk factors for endometrial hyperplasia or cancer are present. Abnormal uterine bleeding can also be a sign of endometrial cancer in premenopausal women, who comprise 20% of cases of endometrial cancer.17 The American College of Obstetricians and Gynecologists (ACOG) recommends that women with abnormal uterine bleeding be evaluated for endometrial cancer if they are older than 45 years, or if they are younger than 45 years and have a history of unopposed estrogen exposure.2,3,19 Evaluation can be done with endometrial tissue sampling or ultrasonography.19
Physical Examination
There are few physical examination findings in women with endometrial cancer. A pelvic examination should be performed to evaluate for other sources of abnormal bleeding, such as the vagina or cervix. The uterus and adnexa should be palpated for unusual masses. Abnormal physical examination findings may be suggestive of more advanced disease.
Laboratory Evaluation
There are no specific laboratory tests for the evaluation of endometrial cancer. Laboratory tests should include a pregnancy test in patients of childbearing age. A complete blood count and prothrombin time and partial thromboplastin time may also be considered for patients with heavy bleeding. Papanicolaou smears are not a required part of the evaluation, but occasionally a Pap smear result can suggest endometrial cancer (i.e., atypical glandular cells).
Diagnostic Studies
Most guidelines recommend either transvaginal ultrasonography or endometrial biopsy as the initial study for the evaluation of endometrial cancer.6,17–19 The American College of Radiology Appropriateness Criteria include tables outlining the preferred imaging studies for the evaluation of abnormal vaginal bleeding, including postmenopausal bleeding, and the pretreatment evaluation and follow-up of endometrial cancer (https://acsearch.acr.org/list). The type of initial study depends on the availability of options and their level of invasiveness, and patient and physician preference.
TRANSVAGINAL ULTRASONOGRAPHY
Transvaginal ultrasonography is often the initial diagnostic study of choice when evaluating for endometrial cancer because of its availability, cost-effectiveness, and high sensitivity.17 Transvaginal ultrasonography can be used to measure endometrial thickness. There is some uncertainty regarding the optimal cutoff for endometrial thickness. Several meta-analyses that have used a cutoff measurement of 5 mm or less had a 96% sensitivity and a posttest probability of 2.5% for endometrial cancer in postmenopausal women.20,21 A recent ACOG committee opinion notes that the cutoff value for a normal transvaginal ultrasonography result should be 4 mm or less.18 Postmenopausal patients with endometrial thickness greater than 5 mm should be evaluated with a tissue sample, especially if bleeding is present. The American College of Radiology uses a cutoff of 5 mm or less.17 The optimal cutoff for evaluating premenopausal women has not been defined, but recommendations include a cutoff of 16 mm or less. In all patients, if bleeding persists despite a normal transvaginal ultrasonography result, a tissue biopsy is warranted.2
ENDOMETRIAL SAMPLING
The definitive diagnosis of endometrial cancer requires an endometrial tissue sample.6 Curettage has been considered the preferred method for obtaining a tissue sample, but the newer Pipelle method offers an alternative.2,22 When an adequate sample is obtained, the Pipelle method has high diagnostic accuracy, with a positive predictive value of 81.7% and a negative predictive value of 99.1%.6 However, adequate samples can be difficult to obtain using the Pipelle method. One study showed that only 34% of patients had an adequate sample.22 This percentage rose to 60% when evaluating women with an endometrial thickness of at least 5 mm. If an adequate sample cannot be obtained, a referral for dilation and curettage should be considered. Additional evaluation is needed if symptoms persist despite a benign biopsy result.6
SALINE INFUSION SONOHYSTEROGRAPHY
Saline infusion sonohysterography can also be used to evaluate the endometrial cavity. This study technique uses saline infused into the endometrial cavity, followed by ultrasonography to allow better visualization of structural changes, particularly when patients have focal irregularities such as polyps, submucosal fibroids, or endometrial hyperplasia.17 Saline infusion sonohysterography is rarely used, but it can be considered when endometrial biopsy or transvaginal ultrasonography is inadequate.17
HYSTEROSCOPY
Hysteroscopy is commonly used to evaluate abnormal uterine bleeding and offers direct visualization of the endometrial cavity.2 Hysteroscopy can be performed in conjunction with a focal biopsy or curettage. A systematic review found that hysteroscopy had a sensitivity of 99.2% and specificity of 86.4% in the diagnosis of endometrial cancer.23
ADDITIONAL DIAGNOSTIC IMAGING
Magnetic resonance imaging may be able to provide additional information on endometrial thickening or structural abnormalities such as fibroids or adenomyosis when transvaginal ultrasonography is not adequate and saline infusion sonohysterography is not tolerated.17 Computed tomography and positron emission tomography are generally not useful in the initial evaluation.17
Treatment
The International Federation of Gynecology and Obstetrics tumor-node-metastasis staging system of endometrial cancer was updated in 2009 and appears better able to predict prognosis compared with the previously published 1988 system.24,25 Major changes to the new system included combining former stages IA and IB, elimination of stage IIA, and stratification of stage IIIC into pelvic nodes only or para-aortic nodal involvement26 (Table 15,24–26 ).
| Stage | Description | Treatment options | Five-year survival (%) | ||
|---|---|---|---|---|---|
| I | Tumor limited to uterine corpus |
| |||
| IA | Tumor limited to endometrium or < 50% myometrial invasion | 90 | |||
| IB | ≥ 50% myometrial invasion | 78 | |||
| II | Tumor invades cervix but not beyond the uterus |
| 74 | ||
| III | Local and/or regional spread |
| |||
| IIIA | Tumor involves serosa and/or adnexa | 56 | |||
| IIIB | Vaginal or parametrial involvement | 36 | |||
| IIIC | Metastases to pelvic and/or para-aortic lymph nodes |
| |||
| IIIC1 | Regional lymph node metastasis to pelvic lymph node | 57 | |||
| IIIC2 | Regional lymph node metastasis to para-aortic lymph nodes with or without pelvic lymph nodes | 49 | |||
| IV | Intra-abdominal or extra-abdominal metastasis |
| |||
| IVA | Invasion of bladder or bowel mucosa | 22 | |||
| IVB | Distant metastasis (including intra-abdominal and inguinal lymph node spread) | 21 | |||
ENDOMETRIAL HYPERPLASIA
Management of endometrial cancer is broken down into surgical and nonsurgical therapies. All patients with endometrial hyperplasia should have testing to rule out concurrent adenocarcinoma.
The definitive treatment for complex atypical endometrial hyperplasia is hysterectomy. Surgical options include abdominal and minimally invasive procedures such as laparoscopy. Hysterectomy can be performed with or without bilateral salpingo-oophorectomy.27 ACOG does not recommend supracervical procedures as treatment because these procedures can leave behind residual disease.28 Abdominal surgery is associated with more pain, longer recovery, and longer hospital stay compared with laparoscopy.29 Additional procedures may be warranted if carcinoma is identified. Lymphadenectomy at the time of surgery is not recommended, as long as there are no intra-abdominal findings suggestive of invasive processes. Most patients with endometrial hyperplasia will not have carcinoma.27
Patients with low-risk endometrial hyperplasia (without atypia) or multiple comorbidities precluding surgery, and those who desire continued fertility, can be treated with nonsurgical options. The most common treatment option is progesterone therapy to stabilize the disease and prevent progression to endometrial cancer.
Use of the levonorgestrel-releasing intrauterine system and oral progesterone (e.g., medroxyprogesterone [Provera], 10 mg daily for 10 to 14 days per month) for the treatment of low- to medium-risk endometrial hyperplasia showed a reduction in hyperplasia six months after treatment.27,30 The optimal route, dose, and duration of therapy have not been well defined. General consensus is to treat patients for six months, with tissue samples obtained every three months to evaluate for disease regression. Multiple endometrial samplings in the posttreatment surveillance period have also been recommended.27
ENDOMETRIAL CANCER
Surgical Approaches. The mainstay of treatment is total hysterectomy with bilateral salpingo-oophorectomy, para-aortic and pelvic lymphadenectomy, and pelvic washing to stage the disease. Laparoscopy has been associated with fewer postoperative complications than laparotomy. Vaginal hysterectomy is generally not recommended because it precludes abdominal survey and lymphadenectomy.3 Most patients who have endometrial cancer will have stage I carcinoma. Need for further treatment is based on intraoperative and histologic findings.2
Adjuvant Radiotherapy. Radiation therapy does not affect overall survival in patients with low-grade carcinoma. It is associated with a reduction in quality of life and increased morbidity when used in patients with low-risk endometrial cancer.3,32 Radiation therapy is an option for patients who are medically inoperable.33
Chemotherapy and Hormone Therapy. Cytoreduction therapy (debulking with surgery and chemotherapy or radiation) appears to improve survival time in patients with intra-abdominal disease by increasing survival and decreasing recurrence.34 Evidence to support the use of adjuvant progesterone therapy to prevent endometrial cancer recurrence is lacking.35 Progesterone is a treatment option for patients with stage I endometrial cancer who wish to preserve fertility.36 There is a 30% chance that the patient whose biopsy noted a grade I carcinoma may have a grade II or III carcinoma instead.37 Patients should be counseled on immediate hysterectomy once childbearing is completed.
Prognosis
Survival is based on the stage and histology of the diagnosis. Most patients with stage I and II endometrial cancer will have a favorable prognosis, whereas patients with stage III or IV endometrial cancer will have a worse likelihood of survival24 (Table 15,24–26 ). Posttreatment surveillance is recommended for detection of recurrent disease. The Society of Gynecologic Oncology recommends follow-up symptom surveillance and pelvic examinations every three to six months for two years posttreatment, then every six months for three years, and annually thereafter.3
Data Sources: Searched Cochrane databases, DynaMed, PubMed, PEPID, Clinical Evidence, National Guideline Clearinghouse, Essential Evidence Plus, UpToDate, and OVID using key terms endometrial cancer, tamoxifen, cancer, diagnosis, endometrial hyperplasia, biopsy, and treatment. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. Search dates: November 1, 2014, through February 20, 2015; August 1, 2015; and December 1, 2015.
The views expressed in this article are those of the authors and do not reflect the official policy of the Department of the Army, the Department of Defense, or the U.S. government.
