This is a corrected version of the article that appeared in print. Figure 2 has been updated.
Am Fam Physician. 2017;96(8):515-522A
AAFP Resource: See related resources from the American Academy of Family Physicians on alcohol misuse.
Patient information: A handout on this topic is available at https://familydoctor.org/condition/fetal-alcohol-syndrome.
Author disclosure: No relevant financial affiliations.
Fetal alcohol syndrome (FAS) and fetal alcohol spectrum disorders (FASD) result from intrauterine exposure to alcohol and are the most common nonheritable causes of intellectual disability. The percentage of women who drink or binge drink during pregnancy has increased since 2012. FAS is commonly missed or misdiagnosed, preventing affected children from receiving needed services in a timely fashion. Diagnosis is based on the presence of the following clinical features, all of which must be present: prenatal and/or postnatal growth retardation, facial dysmorphology, central nervous system dysfunction, and neurobehavioral disabilities. FASD is a broader diagnosis that encompasses patients with FAS and others who are affected by prenatal alcohol exposure but do not meet the full criteria for FAS. Management is multidisciplinary and includes managing comorbid conditions, providing nutritional support, managing behavioral problems and educational difficulties, referring patients for habilitative therapies, and educating parents. The Centers for Disease Control and Prevention and other organizations recognize no safe amount of alcohol consumption during pregnancy and recommend complete abstinence from alcohol. All women should be screened for alcohol use during preconception counseling and prenatal care, and alcohol use should be addressed with brief interventions.
WHAT IS NEW ON THIS TOPIC: FETAL ALCOHOL SPECTRUM DISORDERS
According to the Centers for Disease Control and Prevention, the percentage of pregnant women who consume alcohol increased from 7.6% in 2012 to 10.2% in 2015, and the number of pregnant women reporting binge drinking (at least four alcoholic beverages at once) increased from 1.4% to 3.1%.
A study demonstrated that more than one-half of children with fetal alcohol spectrum disorders do not consume the recommended dietary allowance of fiber, calcium, or vitamins D, E, and K.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| The diagnosis of fetal alcohol syndrome and partial fetal alcohol syndrome is based on defined clinical characteristics and does not require confirmed alcohol use during pregnancy. | C | 1 |
| Neurobehavioral testing should be conducted in all children with suspected fetal alcohol spectrum disorders when feasible. Comprehensive evaluation may not be possible using conventional assessment tools until after three years of age. | C | 1 |
| Contraception should be offered to women of childbearing age who drink. If they desire pregnancy, abstinence from alcohol should be recommended. | C | 44 |
| Pregnant women should be screened for alcohol use. This can be done using the TACER-3 tool. | C | 42, 45, 46 |
According to the Centers for Disease Control and Prevention, the percentage of pregnant women who consume alcohol increased from 7.6% in 2012 to 10.2% in 2015, and the number of pregnant women reporting binge drinking (four or more alcoholic beverages at once) increased from 1.4% to 3.1%.3,4 These trends are concerning because alcohol is the most common teratogen, and FASD is the most common cause of nonheritable intellectual disability.5 Binge drinking is associated with the development of behavioral problems and physical deformities.6
Although there is wide variation in the estimated prevalence of FAS/FASD, FAS is thought to occur in 0.3 to 0.8 per 1,000 children in the United States and in 2.9 per 1,000 globally.7,8 The prevalence of FASD is estimated at 33.5 per 1,000 children in the United States and 22.8 per 1,000 globally.8 In the United States, FASD is least prevalent in Hispanic children and most prevalent in Native Americans and Alaska Natives.4 FAS is diagnosed at an average age of 48.3 months9; however, it is commonly missed or misdiagnosed, preventing affected children from receiving needed services in a timely fashion.
FASD carries a significant economic burden. Children with FAS who are enrolled in Medicaid have annual mean medical expenses nine times higher than those for children without FAS, equating to a median annual expenditure of $6,670 per child (vs. $518 for those without FAS).10
Diagnosis
Any child who was exposed to alcohol pre-natally or presents with growth retardation, facial dysmorphology, central nervous system dysfunction, or neurobehavioral disabilities—the key manifestations of FASD—should prompt consideration of FASD.11 The assessment and diagnosis require a multidisciplinary team (Table 11,12 ) and should include neuropsychological assessment.1

| Audiologist |
| Cardiologist |
| Developmental pediatrician |
| Developmental therapist |
| Family therapist |
| Nephrologist |
| Neurologist |
| Occupational therapist |
| Ophthalmologist |
| Physical therapist |
| Play therapist |
| Primary care physician |
| Psychiatrist |
| Psychotherapist |
| Sensory integration therapist |
| Social worker |
| Special education teachers |
| Speech-language pathologist |
Diagnosis begins with assessment of prenatal alcohol exposure, including quantity of alcohol consumed per occasion, frequency of use, and timing of consumption during pregnancy. Prenatal alcohol exposure is defined as at least one of the following documented findings: (1) six or more drinks per week for two or more weeks during pregnancy; (2) three or more drinks per occasion on two or more occasions during pregnancy; (3) alcohol-related social or legal problems around the time of pregnancy; (4) intoxication during pregnancy documented by blood, breath, or urinary alcohol testing; (5) positive test for alcohol exposure biomarkers during pregnancy (fatty acid ethyl esters, phosphatidylethanol, and ethyl glucuronide in maternal hair, fingernails, urine, or blood, or in placenta or meconium); (6) increased prenatal risk associated with alcohol use during pregnancy as assessed by a validated screening tool. Documentation includes drinking levels reported by the mother three months before pregnancy recognition or at the time of a positive pregnancy test. Information must be obtained by the mother or a reliable source, such as family member, social service agency, or medical record.1
Exposure to other teratogens should also be assessed, because women who consume alcohol during pregnancy are more likely to use other drugs.1 The diagnostic criteria for FAS or PFAS do not require confirmed alcohol use if characteristic findings are present.1,11 However, a confirmed absence of alcohol exposure rules out the diagnoses. Confirmation of alcohol exposure is required for diagnosis of alcohol-related neurodevelopmental disorder and alcohol-related birth defects.1
KEY DIAGNOSTIC CRITERIA
As previously noted, FASD comprises four distinct categories: FAS, PFAS, alcohol-related neurodevelopmental disorder, and alcohol-related birth defects. Each category is distinguished by the presence or absence of characteristic facial dysmorphology, growth retardation, central nervous system dysfunction, and neurobehavioral disabilities (Table 2).1
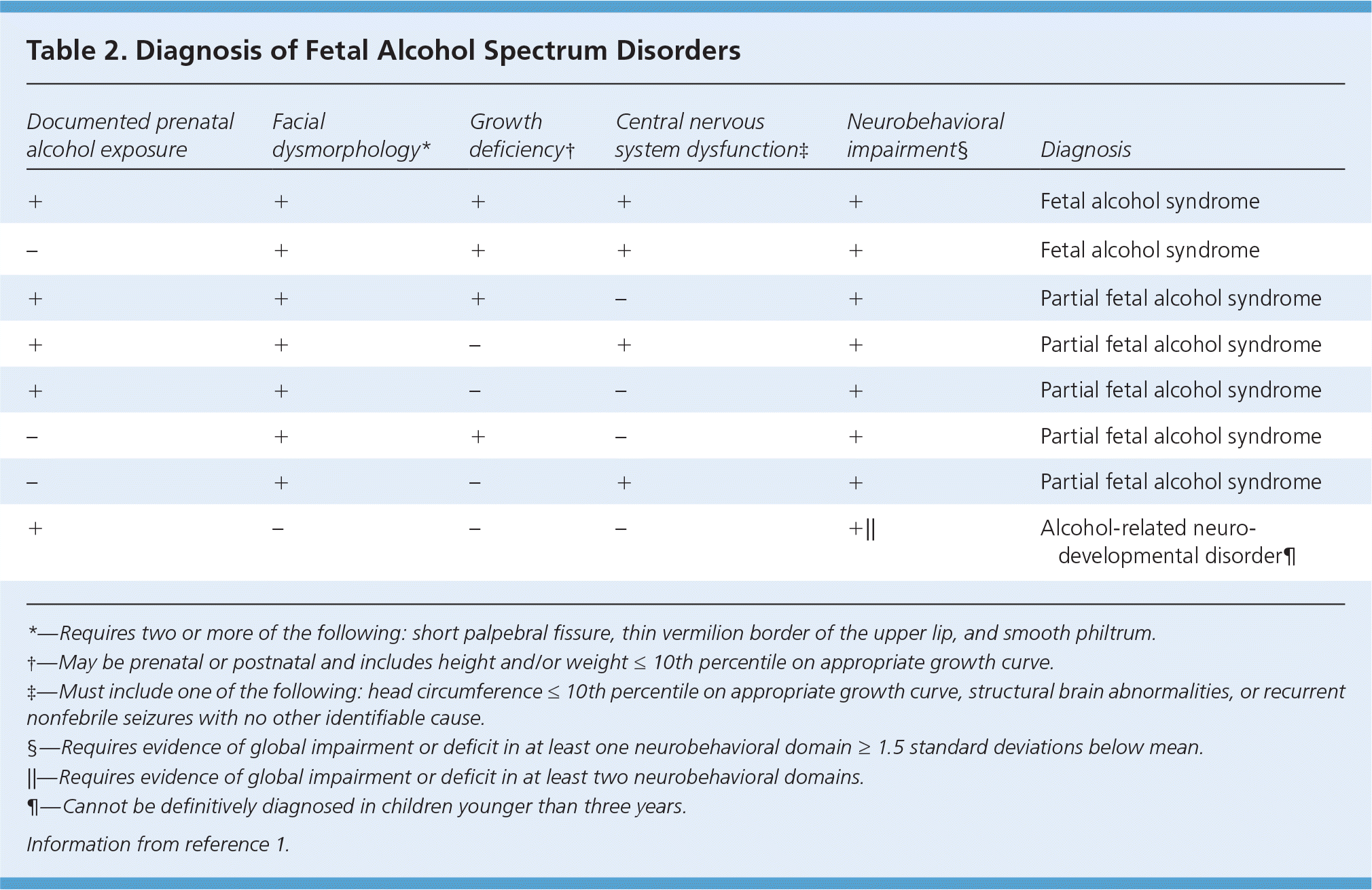
| Documented prenatal alcohol exposure | Facial dysmorphology* | Growth deficiency† | Central nervous system dysfunction‡ | Neurobehavioral impairment§ | Diagnosis |
|---|---|---|---|---|---|
| + | + | + | + | + | Fetal alcohol syndrome |
| – | + | + | + | + | Fetal alcohol syndrome |
| + | + | + | – | + | Partial fetal alcohol syndrome |
| + | + | – | + | + | Partial fetal alcohol syndrome |
| + | + | – | – | + | Partial fetal alcohol syndrome |
| – | + | + | – | + | Partial fetal alcohol syndrome |
| – | + | – | + | + | Partial fetal alcohol syndrome |
| + | – | – | – | +|| | Alcohol-related neurodevelopmental disorder¶ |
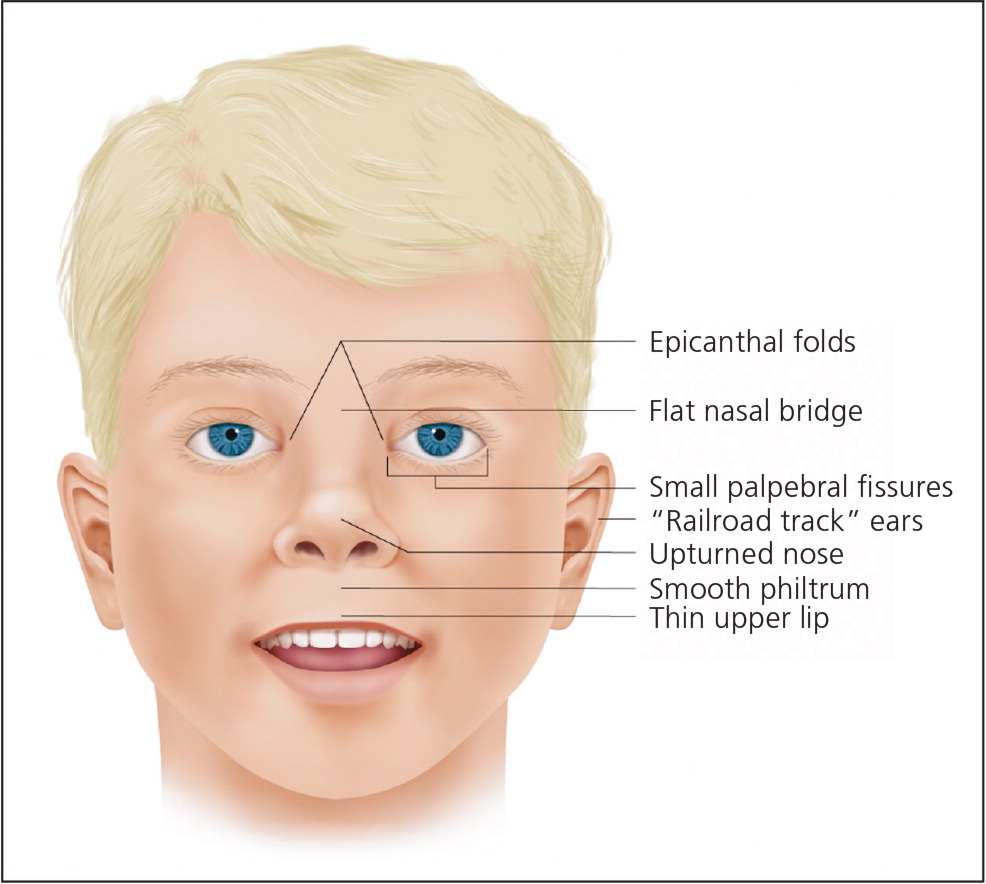
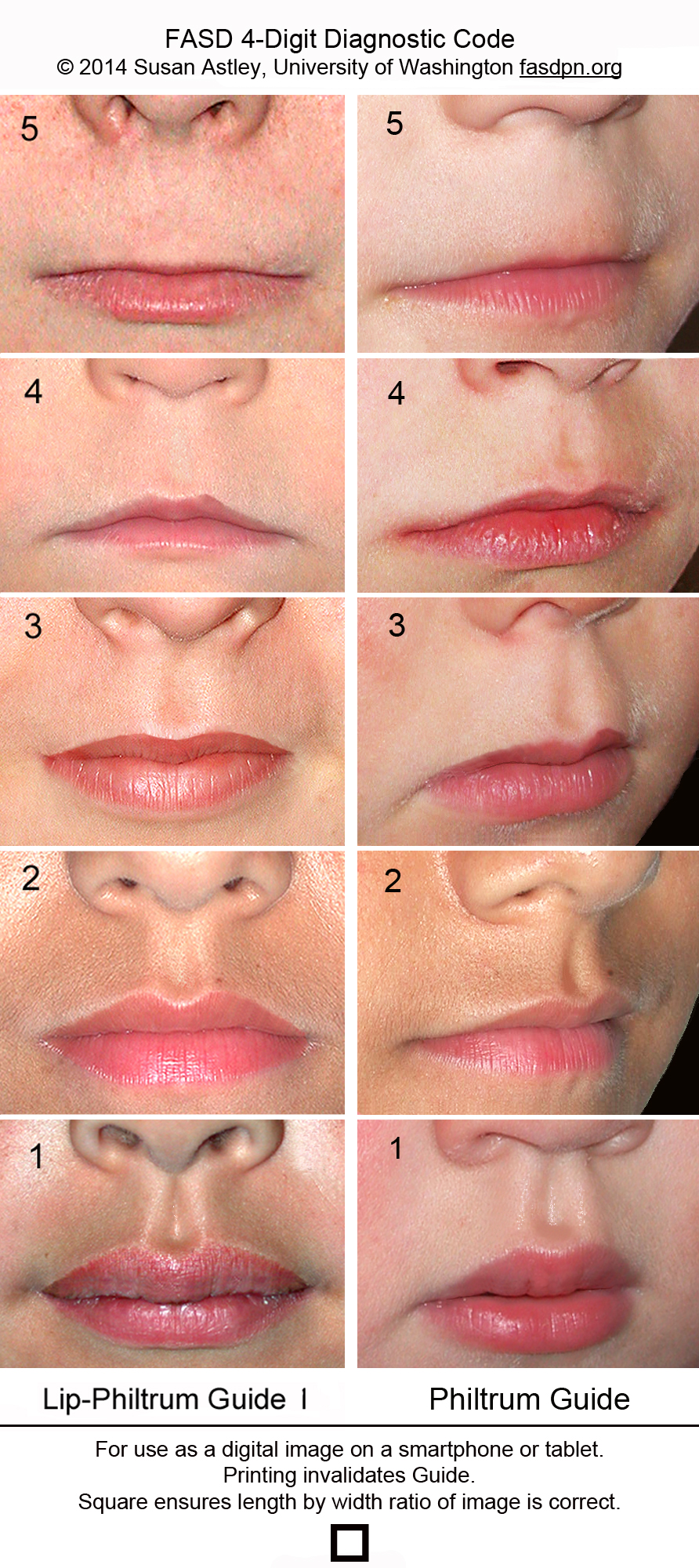
Characteristic facial dysmorphology associated with FASD includes short palpebral fissures (10th percentile or less for age and racial norms), a thin vermilion border of the upper lip, and a smooth philtrum1 (Figure 113 ). Two of the three characteristic features are required for the diagnosis of FAS or PFAS. Palpebral fissures can be measured using a small plastic ruler, noting the distance between the endocanthion (where the eyelids meet medially) and exocanthion (where they meet laterally). The ruler should be angled to follow the curve of the zygoma.1 The presence of a thin vermilion border and smooth philtrum is scored objectively using the lip-philtrum scoring guide (Figure 2).14 Scores of 4 or 5 are consistent with FAS or PFAS.
Growth retardation is defined as the 10th percentile or less using height and weight measurements on standard growth curves.1 For central nervous system dysfunction to qualify as consistent with FASD, it must include deficient brain growth, abnormal structure, or abnormal neurophysiology. This can be documented as a head circumference in the 10th percentile or less on appropriate growth curves, structural brain abnormalities, or recurrent nonfebrile seizures with no other identifiable cause.1 Magnetic resonance imaging has identified structural brain abnormalities in children with FASD (e.g., temporal lobe asymmetry, change in size or shape of corpus callosum, cerebellum, or basal ganglia), and it may be used in the evaluation of suspected FASD; it can also be helpful if there is a question about the differential diagnosis.1,15–17
Neurobehavioral disabilities in FASD include deficient global intellectual ability and cognition, and poor behavior, self-regulation, and adaptive skills. These domains should be measured using standardized testing, which often cannot be administered until after three years of age. A deficiency on these tests is characterized by scores of at least 1.5 standard deviations below the mean.1 Alcohol-related neurodevelopmental disorder is diagnosed with documented prenatal alcohol exposure and neurobehavioral impairment in at least two domains in the absence of other defining characteristics for FAS.
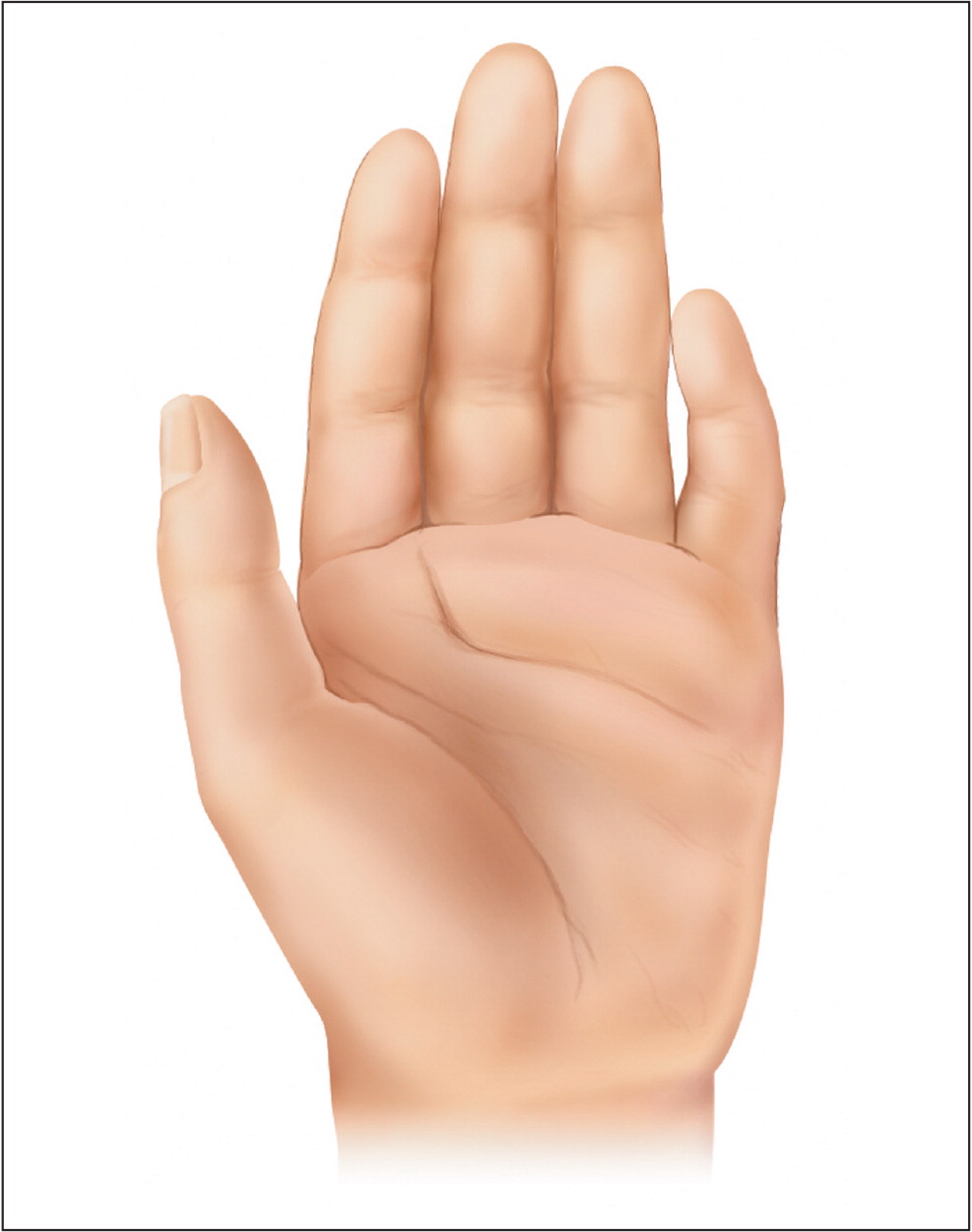
Although they are not included in the diagnostic criteria for FAS or PFAS, multiple congenital abnormalities associated with prenatal alcohol exposure have been described for nearly every organ system (Table 3).15,18–21 In the absence of defining criteria for FAS or PFAS, documented prenatal alcohol exposure and the presence of one or more major malformations known to result from prenatal alcohol exposure are diagnostic for alcohol-related birth defects1 (eTable A, Figure 313).

| System | Condition |
|---|---|
| Auditory | Chronic serous otitis media, conductive and/or neurosensory hearing loss |
| Cardiac | Aberrant great vessels, atrial septal defects, ventricular septal defects |
| Gastrointestinal | Enteric neuropathy |
| Musculoskeletal | Camptodactyly, clinodactyly (Figure 3), flexion contractures, hypoplastic nails, radioulnar synostosis, scoliosis, spinal malformations |
| Neurologic | Microcephaly, seizure disorder, spinal cord abnormalities, structural brain abnormalities (including corpus callosum, cerebellum, caudate, and hippocampus) |
| Ophthalmologic | Ptosis, retinal malformation, strabismus, visual impairment |
| Orofacial | Cleft lip, cleft palate |
| Psychiatric/ neurobehavioral | Attention-deficit/hyperactivity disorder, conduct disorder, intellectual disability, language disorders, learning disabilities, mood disorders, oppositional defiant disorder, substance use disorders |
| Renal | Aplastic/dysplastic/hypoplastic kidneys, horseshoe kidney, hydronephrosis, ureteral duplications |

| Documented prenatal alcohol exposure | |
| At least 1 of the following specific major malformations known to be the result of prenatal alcohol exposure: | |
| Auditory: conductive and/or neurosensory hearing loss | |
| Cardiac: aberrant great vessels, atrial septal defect, conotruncal heart defects, ventricular septal defect | |
| Musculoskeletal: flexion contractures, radioulnar synostosis, scoliosis, vertebral segmentation defects | |
| Ophthalmologic: optic nerve hypoplasia, ptosis, retinal vascular anomalies, strabismus | |
| Renal: aplastic/dysplastic/hypoplastic kidneys, horseshoe kidney, ureteral duplications | |
Differential Diagnosis
The differential diagnosis for FASD includes a variety of chromosomal abnormalities, exposure to other teratogens, and behavioral and psychiatric diagnoses (Table 4).2,22–28 If the diagnosis is uncertain, the workup should include referral to a developmental pediatrician or geneticist for further evaluation, which may involve a chromosomal microarray, cranial neuroimaging, and cardiac/abdominal ultrasonography.2

| Condition | Cause | Features similar to fetal alcohol syndrome | Distinguishing features from fetal alcohol syndrome |
|---|---|---|---|
| Aarskog syndrome | X-linked recessive, often mutations in FGD1, although others unknown | Broad philtrum, intellectual and neurobehavioral disabilities, small nose with anteverted nares, wide-spaced eyes | Brachydactyly, crease below lower lip, dental eruption problems, downward-slanting palpebral fissures, shawl scrotum (scrotum folds around penis), short stature that resolves with puberty, widow's peak |
| Bloom syndrome | Autosomal recessive chromosomal instability caused by mutation in BLM | Short stature with mild microcephaly, variably impaired intellectual ability | Café au lait spots; facial telangiectasia erythema; keel-shaped face; predisposition to early cancer, infertility, and immunodeficiency; sparse subcutaneous adipose tissue |
| Cornelia de Lange (Brachmann-de Lange) syndrome | Autosomal dominant from spontaneous mutations in NIPBL, RAD21, and SMC3, or X-linked dominant with mutations in HDAC8 or SMC1A | Anteverted nares, depressed nasal bridge, growth impairment, hearing loss, intellectual disability, microcephaly, short stature, smooth philtrum, thin vermilion border | Arched eyebrows that meet in the middle (synophrys), downturned mouth, high arched palate, hypertrichosis, long eyelashes, short limbs |
| Dubowitz syndrome | Unknown; suspected autosomal recessive | Neurobehavioral disabilities (hyperactivity, impulsivity, and inattentiveness), epicanthal folds, intellectual disability, microcephaly, short palpebral fissures, short stature, wide-spaced eyes | Broad nasal tip, cryptorchidism, eczema-like skin disorder, high-pitched voice, shallow supraorbital ridge with nasal bridge near level of forehead |
| Fetal hydantoin syndrome | Prenatal exposure to phenytoin (Dilantin) | Depressed nasal bridge, growth deficits, occasional intellectual disability, wide-spaced eyes | Genitourinary defects, hirsutism, hypoplastic fingertips, low hairline, orofacial clefts, short neck, short nose with bowed upper lip |
| Fetal valproate syndrome | Prenatal exposure to valproate (Depacon) | Anteverted nares, epicanthal folds, long philtrum, thin vermilion border, wide-spaced eyes | Cardiac malformations, high forehead, infraorbital crease, neural tube defects, small mouth |
| Noonan syndrome | Autosomal dominant, often mutation in PTPN11 | Epicanthal folds, intellectual disability, low nasal bridge, short stature, wide-spaced eyes | Bleeding diathesis, cryptorchidism, downward-slanting palpebral fissures, hypertrophic cardiomyopathy, keratoconus, low posterior hairline, pectus excavatum, protruding upper lip, pulmonary stenosis, webbed neck, wide mouth |
| Phenylalanine embryopathy | Maternal phenylketonuria | Epicanthal folds, growth impairment, intellectual disability, long philtrum, microcephaly, short palpebral fissures, small nose with anteverted nares, thin vermilion border | Cardiac malformations, hypertonia, prominent glabella, round facies |
| Toluene embryopathy | Prenatal exposure to toluene | Growth deficits, midface hypoplasia, short palpebral fissures, smooth philtrum, thin vermilion border | Bifrontal narrowing of the skull, downturned mouth, ear abnormalities, hair pattern abnormalities, large anterior fontanelle, micrognathia |
| Velocardiofacial syndrome | Autosomal dominant with microdeletion in chromosome 22q11 | Intellectual disabilities, psychiatric disorders, small palpebral fissures | Cardiac malformations, cleft palate, long face with prominent nose, transient neonatal hypocalcemia, weak pharyngeal muscles resulting in hypernasal speech |
| Williams syndrome | Heterozygous 7q11.23 deletion, including elastin gene | Anteverted nares, depressed nasal bridge, epicanthal folds, growth impairment, intellectual disability, long philtrum, short nose, short palpebral fissures | Aortic and pulmonary stenosis, connective tissue disorders, endocrine abnormalities, full lips, hoarse voice, hypertension, periorbital fullness, poor to near-normal language skills, renal abnormalities, stellate pattern of iris, systemic arterial stenosis, wide mouth |
Management
There is no cure for FASD.5 There is a lack of evidence on which to base recommendations for optimal management; therefore, much of the management is based on expert opinion. Treatment consists of providing a medical home for the patient and family, managing comorbid conditions, providing nutritional support, addressing behavioral and emotional problems, arranging referrals for habilitative therapies (therapeutic intervention for those who have never developed a specific skill), coordinating care with a multidisciplinary team, and educating parents (Table 5). Early intervention is necessary to optimize health outcomes.11,29

| American Academy of Pediatrics Fetal Alcohol Spectrum Disorders Program |
| http://www.aap.org/en-us/advocacy-and-policy/aap-health-initiatives/fetal-alcohol-spectrum-disorders-toolkit/ |
| Centers for Disease Control and Prevention |
| https://www.cdc.gov/ncbddd/fasd/ and https://www.cdc.gov/ncbddd/fasd/alcohol-use.html |
| National Organization on Fetal Alcohol Syndrome (also contains resources for teachers) |
| http://www.nofas.org/parents/ |
MANAGING COMORBID CONDITIONS
Children with FASD can have a range of comorbid conditions (Table 3)15,18–21; referrals to members of the multidisciplinary team are based on the specific needs identified. Because hearing and vision impairments are correlated with prenatal alcohol exposure, all children with suspected FASD should have hearing and vision screening.30,31
NUTRITIONAL SUPPORT
Children with FASD are nutritionally and socially vulnerable and may benefit from nutritional education and support. By midchildhood, most of these children have spent, on average, one-fourth of their life with unmet basic needs and one-third of their life with someone who abuses alcohol or drugs.29 One study showed that more than 50% of children with FASD do not consume the recommended dietary allowance of fiber, calcium, or vitamins D, E, and K.32 It is important to regularly assess the child's height, weight, and body mass index and refer for support (e.g., nutritionist, social worker) when nutritional problems are identified.33 Some children will require high-calorie foods and supplements.
MANAGING BEHAVIORAL PROBLEMS
Children with FASD should be monitored and screened for behavioral problems. They have an increased risk of attention-deficit/hyperactivity disorder (40% to 95%),34,35 mood disorders (50%),36 and oppositional defiant disorder (38%).35 Medications can improve hyperactivity and impulsivity, but not symptoms of inattention.37,38 Children with FASD and attention-deficit/hyperactivity disorder or other disruptive behaviors should be referred to a developmental pediatrician, psychologist, and/or psychiatrist. Behavioral interventions such as play therapy, children's friendship training, and specially trained case managers have reasonable evidence of effectiveness, but these resources have variable availability.37
FAMILY SUPPORT
Children with FASD are at increased risk of physical and sexual violence, with 61% experiencing physical or sexual abuse or witnessing domestic violence by 12 years of age.29,39 Sexual abuse should be considered in children who present with inappropriate sexual behaviors. Children with FASD who remain in the care of their biologic mother are more likely to experience family dysfunction and instability (e.g., divorce, unemployment, drug and alcohol use).25,29 Those who are raised in stable homes have improved outcomes and are less likely to be expelled from or drop out of school, be arrested, or develop substance use problems.29 Interventions should be aimed at stabilizing the home environment and improving parent-child interactions.11 Such interventions include parental substance abuse referrals, child discipline courses, parent support groups, and child protective services.
Prognosis
Prognosis varies with the degree of impairment. Persons with FASD are more likely to require special education, receive disability pensions, and be unemployed.40 Those who receive early diagnosis and intervention (before 12 years of age) have significantly better outcomes, including a two- to fourfold reduction in rates of imprisonment and substance abuse.29
Prevention
The Centers for Disease Control and Prevention, the American Academy of Family Physicians, the American Academy of Pediatrics, and the American Congress of Obstetricians and Gynecologists recognize no safe amount of alcohol consumption during pregnancy and recommend complete abstinence.26,41–43 Although many women abstain from alcohol when they learn they are pregnant, some consume alcohol before they find out. Contraception should be offered to women of child-bearing age who drink; if they desire pregnancy, abstinence from alcohol should be recommended.44 The American Congress of Obstetricians and Gynecologists recommends screening women in the first trimester for alcohol use, and Canadian guidelines recommend screening all pregnant women for alcohol use.42,45 A useful screening tool is the TACER-3, which identifies women whose drinking may put their fetus at risk of FASD (Table 6).46

| Component | Positive reply | Score | Question |
|---|---|---|---|
| Tolerance | ≥ 2 drinks* | 2 | How many drinks does it take to make you feel high? |
| Annoyance | Yes | 1 | Has anybody ever annoyed you by complaining about your drinking? |
| Cut down | Yes | 1 | Have you ever felt you ought to cut down on your drinking? |
| Eye-opener | Yes | 1 | Have you ever needed a drink first thing in the morning to get going? |
If alcohol use in pregnancy is identified, physicians should recommend cessation and offer group-based interventions such as Alcoholics Anonymous and alcohol rehabilitation centers.47 Brief interventions that include the patient's partner improve FASD-related birth outcomes and should include assessing maternal understanding of healthy pregnancy behaviors, assisting the mother in setting the goal of abstinence from alcohol, planning alternative behaviors for when the temptation to drink arises, and inviting the partner to find methods to support the mother's abstinence from alcohol.48,49
This article updates a previous article on this topic by Wattendorf and Muenke.13
Data Sources: Sources searched include PubMed (OVID), Evidence Summary from the AFP's editors, Essential Evidence Plus, Cochrane database, and the Agency for Healthcare Research and Quality. Search terms included: fetal alcohol syndrome, fetal alcohol spectrum disorder, alcohol-related birth defects, maternal alcohol consumption, prenatal alcohol exposure. Search dates: February 2016, April 2016, May 2016, June 2016, July 2016, November 2016, and December 2016.