
Am Fam Physician. 2018;97(12):online
Author disclosure: No relevant financial affiliations.
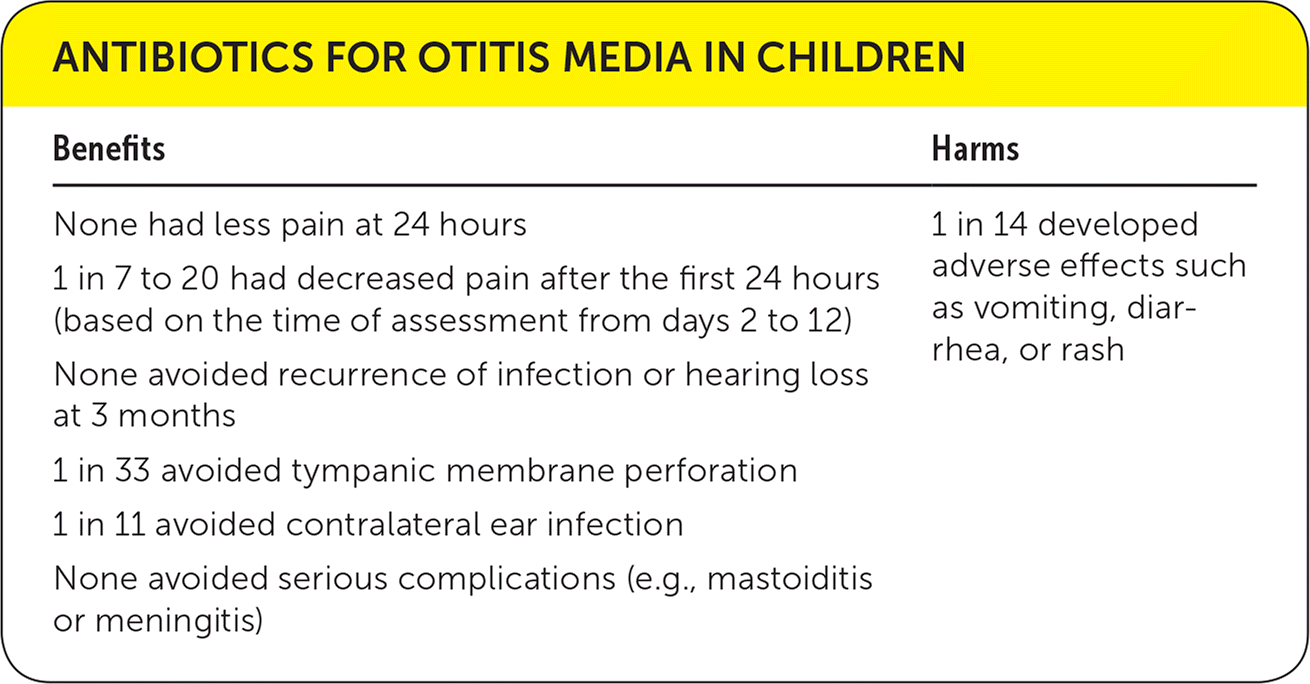
| Benefits | Harms |
|---|---|
| None had less pain at 24 hours | 1 in 14 developed adverse effects such as vomiting, diarrhea, or rash |
| 1 in 7 to 20 had decreased pain after the first 24 hours (based on the time of assessment from days 2 to 12) | |
| None avoided recurrence of infection or hearing loss at 3 months | |
| 1 in 33 avoided tympanic membrane perforation | |
| 1 in 11 avoided contralateral ear infection | |
| None avoided serious complications (e.g., mastoiditis or meningitis) |
Details for This Review
Study Population: Children between two months and 15 years of age from high-income countries enrolled in 13 randomized controlled trials (3,401 participants)1
Efficacy End Points: Pain at various time points (24 hours, two to three days, four to seven days, 10 to 12 days), tympanic membrane (eardrum) perforation, contralateral ear infection (in unilateral ear infections), recurrence of ear infection, hearing loss (decreased hearing) after three months
Harm End Points: Medication adverse effects such as vomiting, diarrhea, or rash; serious consequences of ear infection such as mastoiditis or meningitis
Narrative: Acute otitis media is a disease that most commonly affects young infants and children. The inflammation and edema caused by the infection can manifest as ear pain or ear fullness, typically accompanied by fever, irritability, and decreased activity and feeding. Complications include tympanic membrane perforation with otorrhea, hearing loss, and recurrent otitis media. More severe complications include mastoiditis, cranial nerve palsies, and meningitis.
Currently, there are no universally accepted guidelines pertaining to the use of antibiotics in children diagnosed with otitis media. The guidelines published by the American Academy of Pediatrics recommend antibiotic prescription for children six months and older with severe signs and symptoms of acute otitis media (moderate to severe otalgia, otalgia for 48 hours or more, or temperature of 102.2°F [39°C] or higher).2 For nonsevere unilateral otitis media, the same guidelines recommend antibiotic treatment or close follow-up based on joint decision-making with parents or caregivers. However, treatment patterns for otitis media differ among physicians. Some prescribe antibiotics liberally, whereas others take a more conservative approach by observing patients for worsening symptoms or development of complications, at which point antibiotics are administered. A Cochrane review compared the effectiveness of antibiotics with expectant observation (no treatment) or placebo. The review analyzes the outcomes for antibiotics vs. placebo and antibiotics vs. expectant observation separately.1
The Cochrane review concludes that antibiotics have no early effect on pain in the first 24 hours (relative risk [RR] = 0.89; 95% confidence interval [CI], 0.78 to 1.01), some effect on pain in the days following (one in seven to 20; RR = 0.70; 95% CI, 0.57 to 0.86 for pain reduction after two to three days; RR = 0.76; 95% CI, 0.63 to 0.91 for pain reduction after four to seven days; RR = 0.33; 95% CI, 0.17 to 0.66 for pain reduction after 10 to 12 days compared with placebo), and some beneficial effect on the number of children with tympanic membrane perforation (one in 33; RR = 0.37; 95% CI, 0.18 to 0.76) or episodes of contralateral ear infection (one in 11; RR = 0.49; 95% CI, 0.25 to 0.95), compared with placebo.1
A meta-analysis using patient data from six high-quality randomized controlled trials is also included in the Cochrane review.1 This meta-analysis concludes that the risk of a prolonged course of antibiotics was twice as high for children younger than two years with bilateral acute otitis media than for children two years and older with unilateral acute otitis media, indicating that antibiotics might be beneficial in resolution of symptoms in this particular subgroup.3
Caveats: The most important caveat is that all trials included in the Cochrane review were conducted in high-income countries. Thus, the results might not be generalizable to lower-income countries. In populations with a higher risk of mastoiditis or with lower immunization coverage, antibiotics might prevent serious complications.4
Another notable caveat is the inclusion of patients who received delayed antibiotics in the expectant observation group. In some trials, patients assigned to the observation group were given a prescription but were instructed to withhold antibiotics for 72 hours and to initiate treatment only if symptoms persisted. Delayed antibiotic use might have affected the benefit or harm outcomes in such trials.
Not all trials had specific protocols for pain management (e.g., ibuprofen, acetaminophen). Given that pain reduction was the primary outcome in most trials, the presence or absence of a pain control protocol or of clear instructions for the parents could have influenced the outcomes.
A review of the literature indicates that physicians overdiagnose otitis media, which may result in underestimation of a treatment effect.4 A rational clinical examination systematic review showed that on pneumatic otoscopy, cloudy (adjusted likelihood ratio [LR] = 34; 95% CI, 28 to 42), bulging (adjusted LR = 51; 95% CI, 36 to 73), and distinctly immobile (adjusted LR = 31; 95% CI, 26 to 37) tympanic membranes were the most useful signs of otitis media.5
The methodologic quality of the studies included in the review was deemed to be high, and the evidence for most of the outcomes was considered to be of high quality. For the outcomes of pain at 10 to 12 days, long-term effects, and serious complications, the evidence from the included studies was judged to be of moderate quality because of reporting bias, low sample size, and potential attrition bias.
Factors such as patients being recruited from different practice settings might have contributed to some heterogeneity among the trials. In addition, the duration of antibiotic therapy varied from seven to 14 days in the included trials. Most of the primary outcomes were measured within the first seven days of antibiotic therapy, but a longer duration of antibiotic therapy may have affected secondary outcomes.
Copyright 2018 The NNT Group (theNNT.com). Used with permission.
This series is coordinated by Dean A. Seehusen, MD, MPH, AFP Assistant Medical Editor, and Daniel Runde, MD, from the NNT Group.
A collection of Medicine by the Numbers published in AFP is available at https://www.aafp.org/afp/mbtn.
This review is available from the NNT Group at http://www.thennt.com/nnt/antibiotics-otitis-media-children/.
