
Am Fam Physician. 2022;105(3):327-329
Author disclosure: No relevant financial relationships.
Key Points for Practice
• Start CRC screening at 50 years of age, but consider offering it at 45 years of age for average-risk patients.
• CRC screening beyond age 75 should be individualized based on life expectancy and risk of adverse events.
• Colonoscopy or FIT is recommended for CRC screening because of cost and clinical effectiveness, but other screening tools can be used based on patient preference.
From the AFP Editors
Colorectal cancer (CRC) is the third most common cancer in the United States and ranks second for cancer-related deaths. Between 2011 and 2016, CRC incidence increased at a rate of 1% per year for individuals at average risk, reversing a continuous decrease between 1980 and 2010. The obesity epidemic appears to be the key driver of recent increases. Early detection through screening, removal of precancerous polyps with colonoscopy, and changes in modifiable risk factors were thought to contribute to previous reductions. The American College of Gastroenterology (ACG) released updated guidelines for CRC screening.
When to Screen
The ACG continues to strongly recommend CRC screening between 50 and 75 years of age, and starting screening at 45 years of age is conditionally recommended. Between 1974 and 2013, CRC incidence increased by 51% in people younger than 50 years. Predictive models suggest an increase of 25 life-years per 1,000 persons when screening starts at age 45, although this evidence is very low quality.
When patients are older than 75 years, the decision to screen should be individualized based on patient life expectancy and adverse outcome risk. The benefit of polyp removal is only seen after seven to 10 years, when other medical concerns may affect health more. Older patients have higher adverse event risks, including perforation during colonoscopy, false-positive results, and electrolyte disturbances and dehydration during bowel preparation.
If patients have a first-degree relative with CRC or advanced polyps before 60 years of age or two first-degree relatives with CRC or advanced polyps after age 60, consider screening at 40 years of age or 10 years younger than the youngest affected relative. Up to 10% of adults have this family history, which at least doubles CRC risk. Commencing screening 10 years before diagnosis is a conditional recommendation because of very low quality evidence. Table 1 reviews screening recommendations.
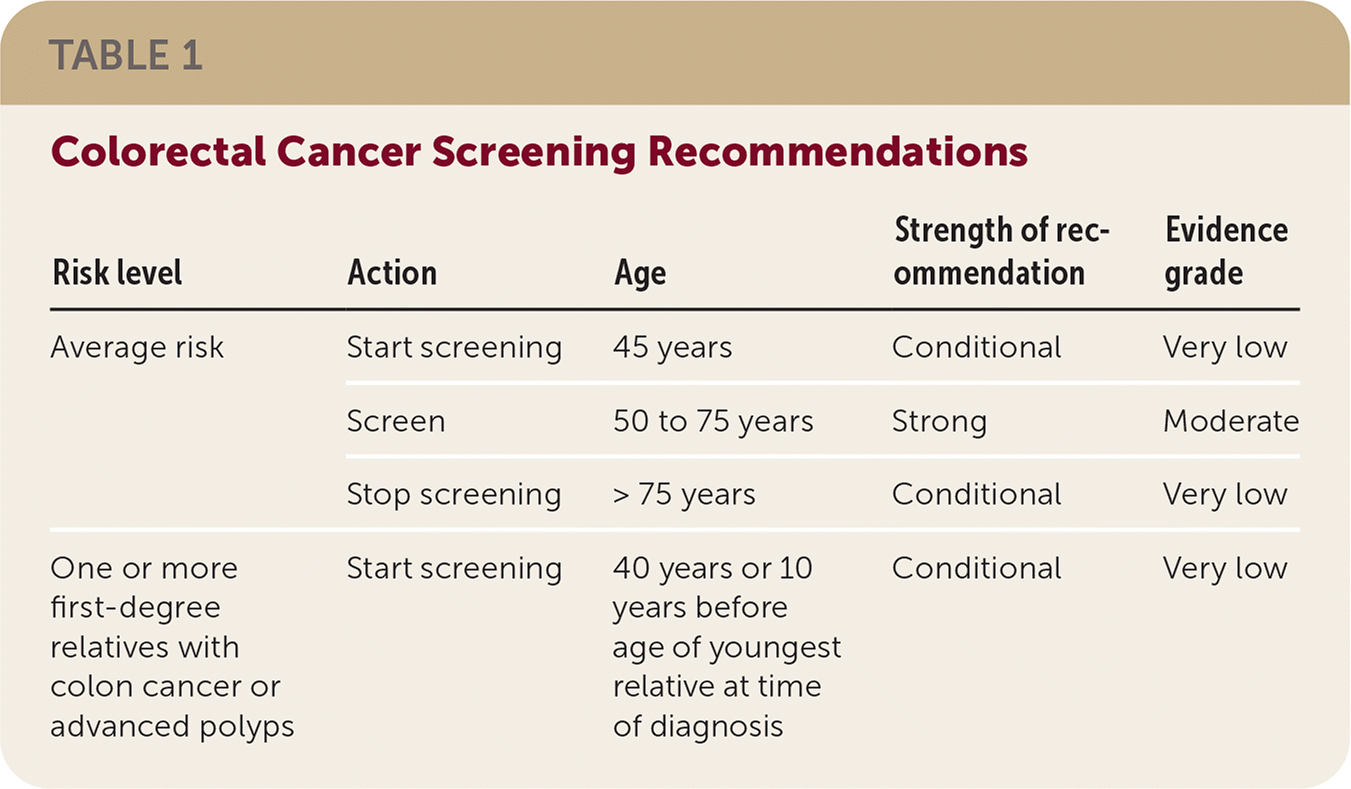
| Risk level | Action | Age | Strength of recommendation | Evidence grade |
|---|---|---|---|---|
| Average risk | Start screening | 45 years | Conditional | Very low |
| Screen | 50 to 75 years | Strong | Moderate | |
| Stop screening | > 75 years | Conditional | Very low | |
| One or more first-degree relatives with colon cancer or advanced polyps | Start screening | 40 years or 10 years before age of youngest relative at time of diagnosis | Conditional | Very low |
Screening Modalities and Frequency
The ACG recommends colonoscopy or fecal immunochemical testing (FIT) as the primary CRC screening method because of cost and clinical effectiveness. Several other two-step screening modalities are acceptable if patient preference increases screening.
Colonoscopy is the only one-step CRC screening modality, because it functions as a diagnostic procedure for the entire colon and a therapeutic procedure when polyps are removed. Colonoscopy has near perfect CRC detection accuracy. The use of colonoscopy for screening reduces CRC incidence by 69% and CRC mortality by 68%. Repeat screening after a normal colonoscopy is recommended every 10 years for average-risk patients and every five years for patients who have a first-degree relative with CRC.
Two-step screening methods consist of a screening modality followed by a colonoscopy for a positive test. Flexible sigmoidoscopy enables diagnostic polyp removal up to the distal colon but requires a full colonoscopy after a positive test. Two-step methods require quality assurance programs to ensure that patients with a positive test result receive a follow-up colonoscopy. CRC screening is performed by stool-based tests, blood tests, or direct visualization.
Stool- and blood-based screening does not require bowel preparation before testing. FIT detects CRC with 91% sensitivity and 90% specificity. Although outcome data are lacking, repeated annual FIT leads to an 80% overall CRC detection rate. FIT has largely replaced fecal occult blood testing (FOBT) because of higher sensitivity for detecting CRC and elimination of the dietary or medication modifications required for FOBT. Although the mortality reduction with FIT is unknown, annual FOBT reduces CRC mortality by 33% and biennial FOBT reduces CRC mortality by 18%. The multitarget stool DNA (mtsDNA) test added to FIT every three years detects CRC with 92% sensitivity and 87% specificity. The specificity of mtsDNA decreases with patient age. The reduced specificity of mtsDNA with FIT leads to more colonoscopies, and the follow-up interval after a positive mtsDNA and negative colonoscopy is uncertain. Septin 9 is a U.S. Food and Drug Administration–approved blood test for individuals 50 years and older that detects CRC with 48% sensitivity and 91% specificity. Because of the low sensitivity, the follow-up interval is unknown, and Septin 9 is not recommended by the ACG.
Two-step visualization tests require bowel preparation. Flexible sigmoidoscopy provides direct visualization of the distal colon and has a 90% to 100% sensitivity for CRC in the distal colon. Use of flexible sigmoidoscopy every five to 10 years reduces CRC mortality by 37%. Computed tomographic colonography every five years detects CRC with a sensitivity between 90% and 100%. It has lower sensitivity for polyps, especially flat and sessile serrated lesions. The colon video capsule performed every five years detects polyps of 6 mm or greater with 81% sensitivity and 93% specificity. Table 2 reviews CRC screening modalities and recommended intervals after normal screening.
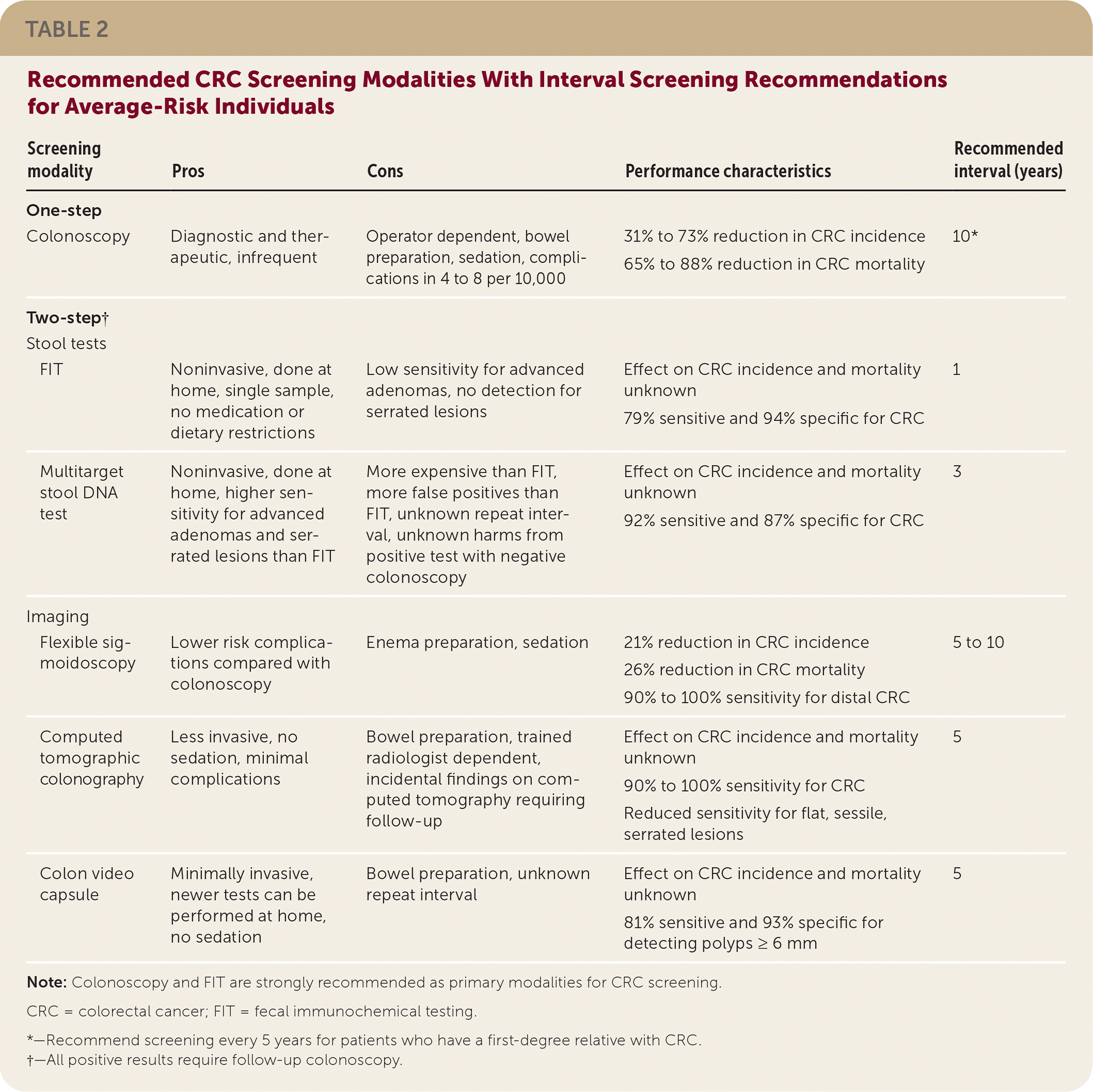
| Screening modality | Pros | Cons | Performance characteristics | Recommended interval (years) |
|---|---|---|---|---|
| One-step | ||||
| Colonoscopy | Diagnostic and therapeutic, infrequent | Operator dependent, bowel preparation, sedation, complications in 4 to 8 per 10,000 | 31% to 73% reduction in CRC incidence 65% to 88% reduction in CRC mortality | 10* |
| Two-step† | ||||
| Stool tests | ||||
| FIT | Noninvasive, done at home, single sample, no medication or dietary restrictions | Low sensitivity for advanced adenomas, no detection for serrated lesions | Effect on CRC incidence and mortality unknown 79% sensitive and 94% specific for CRC | 1 |
| Multitarget stool DNA test | Noninvasive, done at home, higher sensitivity for advanced adenomas and serrated lesions than FIT | More expensive than FIT, more false positives than FIT, unknown repeat interval, unknown harms from positive test with negative colonoscopy | Effect on CRC incidence and mortality unknown 92% sensitive and 87% specific for CRC | 3 |
| Imaging | ||||
| Flexible sigmoidoscopy | Lower risk complications compared with colonoscopy | Enema preparation, sedation | 21% reduction in CRC incidence 26% reduction in CRC mortality 90% to 100% sensitivity for distal CRC | 5 to 10 |
| Computed tomographic colonography | Less invasive, no sedation, minimal complications | Bowel preparation, trained radiologist dependent, incidental findings on computed tomography requiring follow-up | Effect on CRC incidence and mortality unknown 90% to 100% sensitivity for CRC Reduced sensitivity for flat, sessile, serrated lesions | 5 |
| Colon video capsule | Minimally invasive, newer tests can be performed at home, no sedation | Bowel preparation, unknown repeat interval | Effect on CRC incidence and mortality unknown 81% sensitive and 93% specific for detecting polyps ≥ 6 mm | 5 |
Additional Considerations
The accuracy of colonoscopy in detecting adenomas and CRC changes with the performing physician because some physicians identify up to three times as many adenomas as others. Tracking performance is essential to ensure quality. ACG endorses minimal standards that 95% of screening colonoscopies reach the cecum, scope withdrawal times average at least six minutes, and adenomas are found in 25% of studies.
Improving CRC detection in a population requires patient outreach and tracking, which increase screening rates. Out-reach can use repeated patient notifications, clinical decision support tools, or provider recommendations. Patient navigators and peer coaches can reduce barriers. Health systems can send patients FIT kits with return postage to increase screening rates.
Aspirin does not reduce CRC risk in the first 10 years after initiating therapy, but it may reduce incidence with longer use. The use of aspirin is not a substitute for recommended screening.
The opinions and assertions contained herein are those of the authors and are not to be construed as official or as reflecting the views of the Uniformed Services University of the Health Sciences, the U.S. Air Force Medical Department, the Air Force at large, the U.S. Army Medical Department, the Army at large, the Department of the Navy, or the U.S. Department of Defense.
Editor’s Note: These guidelines mainly support the most recent recommendations of the U.S. Preventive Services Task Force (https://www.aafp.org/afp/2021/0900/p295.html) with initiation of CRC screening at 45 years of age and individual determination for those older than 75 years. The ACG adds a preference for colonoscopy and FIT, and includes information that can be helpful with patient discussions. The one-and two-step testing paradigms may be helpful for patients, dovetailing well with data from the BMJ/MAGIC group comparing testing strategies with accuracy and risk of colonoscopy (https://www.aafp.org/afp/2020/0815/p253.html). The ACG guidelines also address the first blood test approved for colon cancer screening, which has insufficient sensitivity and is not recommended.—Michael J. Arnold, MD, Contributing Editor
Guideline source: American College of Gastroenterology
Evidence rating system used? Yes
Systematic literature search described? Yes
Guideline developed by participants without relevant financial ties to industry? Yes
Recommendations based on patient-oriented outcomes? Yes
Published source: Am J Gastroenterol. March 2021;116(3):458–479
