
Am Fam Physician. 2022;105(6):616-624
Author disclosure: No relevant financial relationships.
Stroke accounts for significant morbidity and mortality and is the fifth leading cause of death in the United States, with direct and indirect costs of more than $100 billion annually. Expedient recognition of acute neurologic deficits with appropriate history, physical examination, and glucose testing will help diagnose stroke and rule out mimicking presentations. The National Institutes of Health Stroke Scale should be used to determine stroke severity and to monitor for evolving changes in clinical presentation. Initial neuroimaging is used to differentiate between ischemic and hemorrhagic stroke or other pathologic processes. If a stroke is determined to be ischemic within four and a half hours of last known well or baseline state, determining the patient's eligibility for the administration of intravenous recombinant tissue plasminogen activator is necessary to proceed with informed decision-making for diagnostic workup and appropriate treatment options. Additional evaluation with specialized magnetic resonance imaging studies can help determine if patients can receive recombinant tissue plasminogen activator within nine hours of last known well. Subarachnoid hemorrhage should be considered in the differential diagnosis if the patient presents with rapid onset of severe headache. If radiographic imaging is negative for hemorrhage when there is high suspicion for delayed presentation of stroke, a lumbar puncture should be considered for further evaluation. Patients with cerebellar symptoms should be evaluated with a HINTS (head-impulse, nystagmus, test of skew) examination because it is more sensitive for cerebellar stroke than early magnetic resonance imaging. Additional cerebrovascular imaging should be considered in patients with large vessel occlusions presenting within 24 hours of last known well to assess benefits of endovascular interventions. Once initial interventions have been implemented, poststroke evaluations such as telemetry, echocardiography, and carotid imaging should be performed as clinically indicated to determine the etiology of the stroke.
Risk Factors
Classification of Strokes
Stroke classification is based on the underlying pathologic process and vascular distribution. Appropriate classification of a stroke assists in formulating definitive treatment decisions and communicating potential short- and long-term prognoses. In the United States, approximately 87% of all strokes are ischemic, 10% are intracerebral hemorrhages, and 3% are subarachnoid hemorrhages.1
An ischemic stroke is defined as an episode of neurologic dysfunction secondary to a focal CNS infarction. A silent ischemic infarction is defined as imaging or neuropathologic evidence of an infarction without neurologic dysfunction.
Intracerebral and spontaneous subarachnoid hemorrhages are defined as rapidly developing neurologic dysfunction secondary to the focal accumulation of blood in the brain parenchyma and in the subarachnoid space, respectively, and are not precipitated by trauma. A silent cerebral hemorrhage is similar to a silent ischemic stroke in that both are without clinical symptoms. Imaging shows a focal collection of blood within the brain parenchyma or ventricular system, often presenting in the form of microhemorrhages.2
Clinical Diagnosis
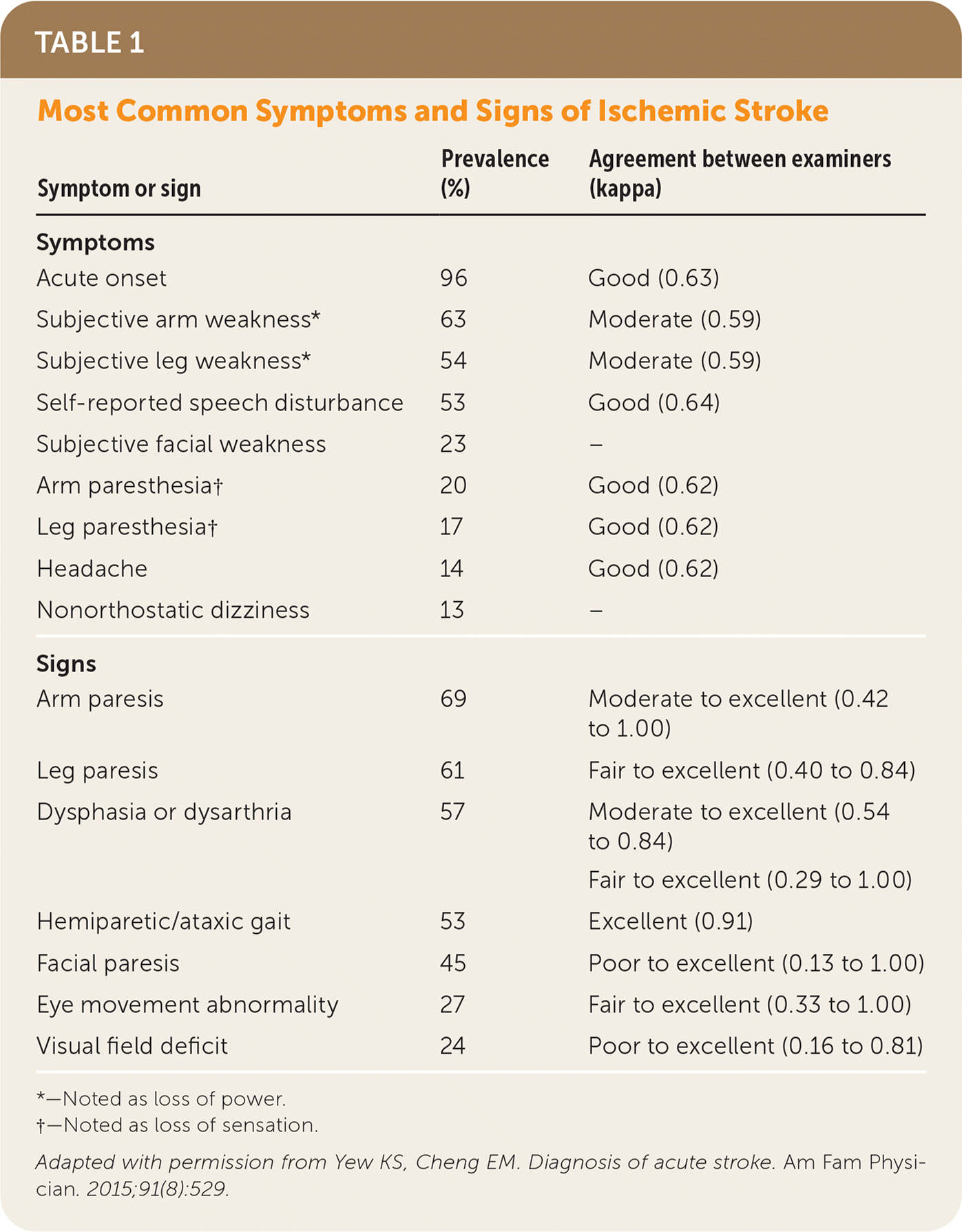
| Symptom or sign | Prevalence (%) | Agreement between examiners (kappa) |
|---|---|---|
| Symptoms | ||
| Acute onset | 96 | Good (0.63) |
| Subjective arm weakness* | 63 | Moderate (0.59) |
| Subjective leg weakness* | 54 | Moderate (0.59) |
| Self-reported speech disturbance | 53 | Good (0.64) |
| Subjective facial weakness | 23 | – |
| Arm paresthesia† | 20 | Good (0.62) |
| Leg paresthesia† | 17 | Good (0.62) |
| Headache | 14 | Good (0.62) |
| Nonorthostatic dizziness | 13 | – |
| Signs | ||
| Arm paresis | 69 | Moderate to excellent (0.42 to 1.00) |
| Leg paresis | 61 | Fair to excellent (0.40 to 0.84) |
| Dysphasia or dysarthria | 57 | Moderate to excellent (0.54 to 0.84) |
| Fair to excellent (0.29 to 1.00) | ||
| Hemiparetic/ataxic gait | 53 | Excellent (0.91) |
| Facial paresis | 45 | Poor to excellent (0.13 to 1.00) |
| Eye movement abnormality | 27 | Fair to excellent (0.33 to 1.00) |
| Visual field deficit | 24 | Poor to excellent (0.16 to 0.81) |
Standardized stroke scoring systems should be used to determine severity of injury and prognosis. The National Institutes of Health Stroke Scale (NIHSS) is the most widely used clinical tool7 (Table 28). NIHSS scores generally reflect the degree of stroke severity: mild (8 or less), moderate (9 to 15), and severe (16 or more).9,10 Stroke scales allow for standardization of communication between clinicians, hospitals, and health care systems to provide an objective means of monitoring for evolving changes in clinical presentation.

| Clinical assessment/scale definition | Score | Clinical assessment/scale definition | Score |
|---|---|---|---|
| Level of consciousness 0 = Alert 1 = Not alert, but arousable 2 = Not alert, obtunded, requires painful stimulation 3 = Unresponsive | ______ | Motor function (leg) 0 = No drift 1 = Drift 2 = Some effort against gravity 3 = No effort against gravity 4 = No movement | |
| Left leg Right leg | ______ ______ | ||
| Level of consciousness questions: patient is asked what month it is and their age 0 = Answers both 1 = Answers one correctly 2 = Answers neither correctly | ______ | Limb ataxia 0 = Absent 1 = Present in one limb 2 = Present in two limbs | ______ |
| Level of consciousness commands: patient is asked to open and close their eyes, then squeeze the nonparetic hand 0 = Performs both tasks correctly 1 = Performs one task correctly 2 = Performs neither task correctly | ______ | Sensory 0 = Normal 1 = Mild to moderate sensory loss 2 = Severe to total sensory loss | ______ |
| Best gaze 0 = Normal 1 = Partial gaze palsy 2 = Forced deviation | ______ | Best language 0 = No aphasia 1 = Mild to moderate aphasia 2 = Severe aphasia 3 = Mute, global aphasia | ______ |
| Vision 0 = No vision loss 1 = Partial hemianopia 2 = Complete hemianopia 3 = Bilateral hemianopia | ______ | Dysarthria 0 = Normal 1 = Mild to moderate dysarthria 2 = Severe dysarthria | ______ |
| Facial palsy 0 = Normal 1 = Minor paralysis 2 = Partial paralysis 3 = Complete paralysis | ______ | Extinction or inattention 0 = No abnormality 1 = Visual, tactile, auditory, spatial, or personal inattention 2 = Profound hemi-inattention or extinction to more than one modality | ______ |
| Motor function (arm) 0 = No drift 1 = Drift 2 = Some effort against gravity 3 = No effort against gravity 4 = No movement | |||
| Left arm Right arm | ______ ______ |
Initial Evaluation
An appropriate workup should be done immediately following history and physical examination to rule out certain conditions that can present similarly to stroke. Stroke mimics include encephalopathies, infection, electrolyte disturbances, psychogenic conditions, and toxicities 11 (Table 38,11). The national standard for identifying stroke mimics is less than 3%; however, they can be identified in a range as low as 1% to as high as 25% based on hospital registries, reflecting that mimics may be difficult to differentiate from acute stroke at presentation.12 Pending diagnostic studies for stroke mimics should not delay initiation of rtPA in eligible patients because the risk of symptomatic intracranial hemorrhage is low.8,9
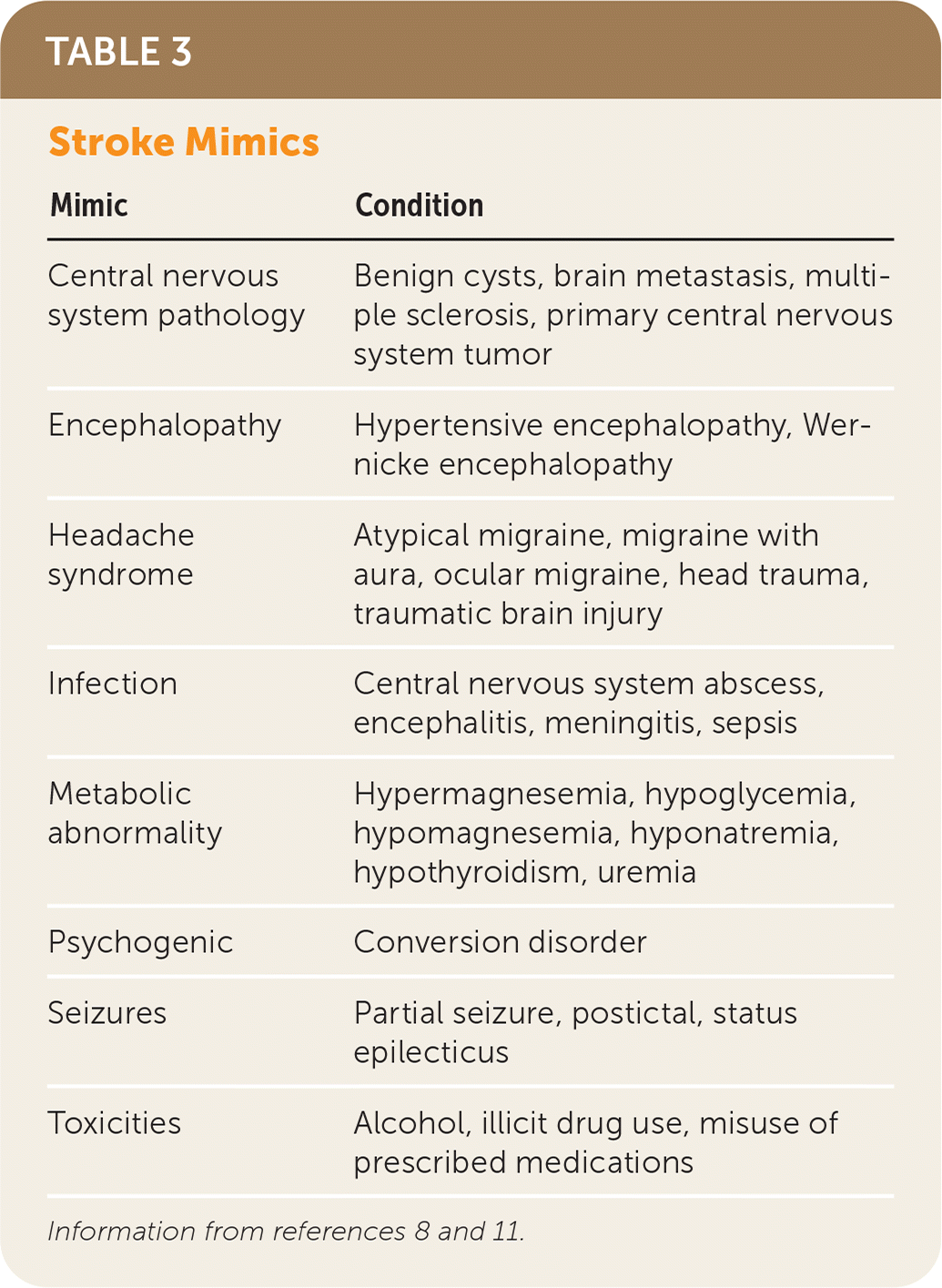
| Mimic | Condition |
|---|---|
| Central nervous system pathology | Benign cysts, brain metastasis, multiple sclerosis, primary central nervous system tumor |
| Encephalopathy | Hypertensive encephalopathy, Wernicke encephalopathy |
| Headache syndrome | Atypical migraine, migraine with aura, ocular migraine, head trauma, traumatic brain injury |
| Infection | Central nervous system abscess, encephalitis, meningitis, sepsis |
| Metabolic abnormality | Hypermagnesemia, hypoglycemia, hypomagnesemia, hyponatremia, hypothyroidism, uremia |
| Psychogenic | Conversion disorder |
| Seizures | Partial seizure, postictal, status epilecticus |
| Toxicities | Alcohol, illicit drug use, misuse of prescribed medications |

| Evaluation | Tests |
|---|---|
| Laboratory | Arterial blood gas* Basic metabolic panel Blood alcohol level* Coagulation panel Complete blood count d-dimer Glucose† Lipid panel Liver enzymes Pregnancy test* Rapid plasma reagin* Toxicology screen* Troponin |
| Procedure | Electrocardiography Electroencephalography* Lumbar puncture* Oxygen saturation |
| Radiologic | Chest radiography* Magnetic resonance imaging of the brain Noncontrast computed tomography of the brain† Transthoracic echocardiography with bubble study* |
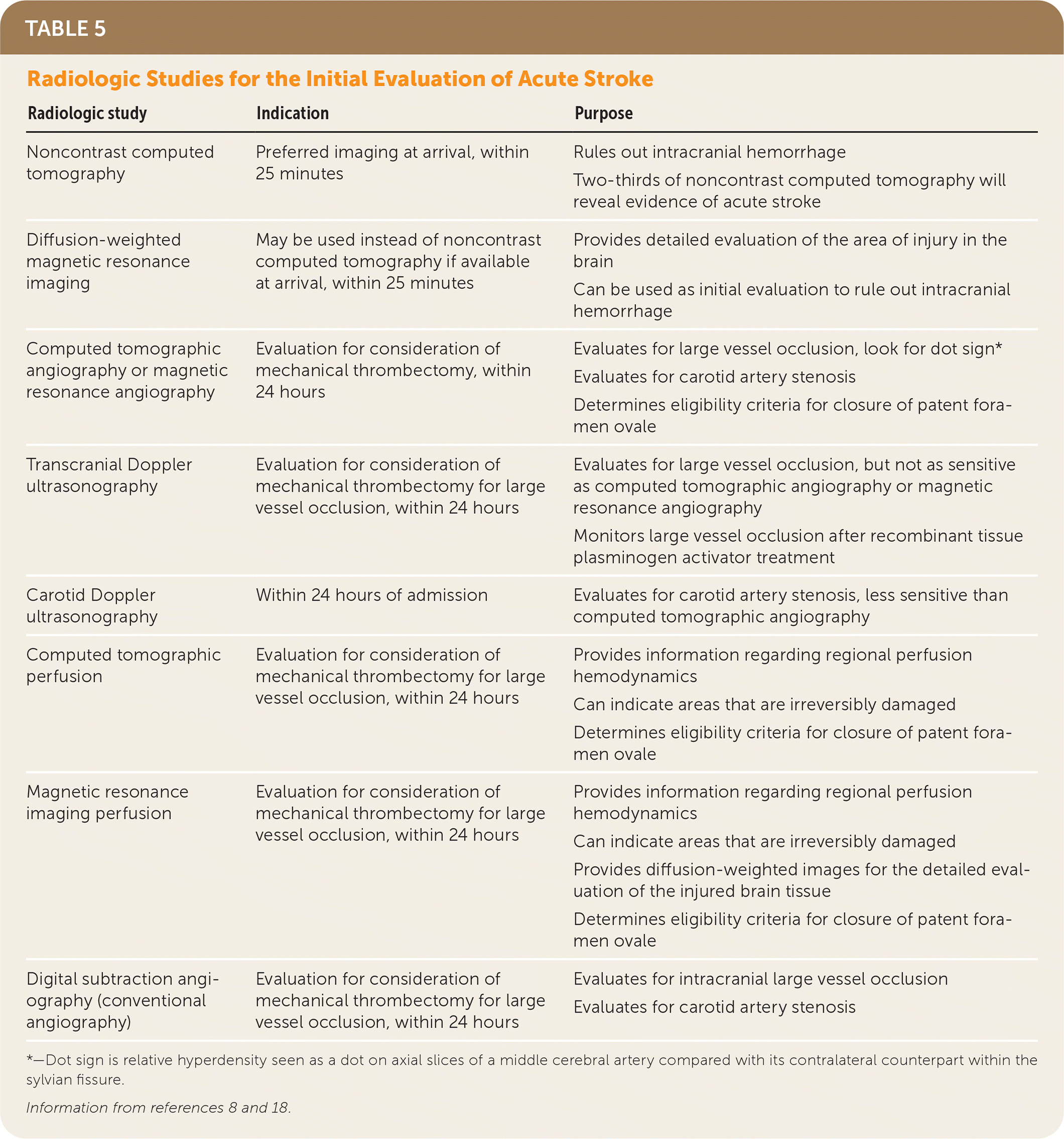
| Radiologic study | Indication | Purpose |
|---|---|---|
| Noncontrast computed tomography | Preferred imaging at arrival, within 25 minutes | Rules out intracranial hemorrhage Two-thirds of noncontrast computed tomography will reveal evidence of acute stroke |
| Diffusion-weighted magnetic resonance imaging | May be used instead of noncontrast computed tomography if available at arrival, within 25 minutes | Provides detailed evaluation of the area of injury in the brain Can be used as initial evaluation to rule out intracranial hemorrhage |
| Computed tomographic angiography or magnetic resonance angiography | Evaluation for consideration of mechanical thrombectomy, within 24 hours | Evaluates for large vessel occlusion, look for dot sign* Evaluates for carotid artery stenosis Determines eligibility criteria for closure of patent foramen ovale |
| Transcranial Doppler ultrasonography | Evaluation for consideration of mechanical thrombectomy for large vessel occlusion, within 24 hours | Evaluates for large vessel occlusion, but not as sensitive as computed tomographic angiography or magnetic resonance angiography Monitors large vessel occlusion after recombinant tissue plasminogen activator treatment |
| Carotid Doppler ultrasonography | Within 24 hours of admission | Evaluates for carotid artery stenosis, less sensitive than computed tomographic angiography |
| Computed tomographic perfusion | Evaluation for consideration of mechanical thrombectomy for large vessel occlusion, within 24 hours | Provides information regarding regional perfusion hemodynamics Can indicate areas that are irreversibly damaged Determines eligibility criteria for closure of patent foramen ovale |
| Magnetic resonance imaging perfusion | Evaluation for consideration of mechanical thrombectomy for large vessel occlusion, within 24 hours | Provides information regarding regional perfusion hemodynamics Can indicate areas that are irreversibly damaged Provides diffusion-weighted images for the detailed evaluation of the injured brain tissue Determines eligibility criteria for closure of patent foramen ovale |
| Digital subtraction angiography (conventional angiography) | Evaluation for consideration of mechanical thrombectomy for large vessel occlusion, within 24 hours | Evaluates for intracranial large vessel occlusion Evaluates for carotid artery stenosis |

| Recommended with likely benefit for patients presenting within three hours of last known well |
| Age ≥ 18 years |
| Concurrent acute ischemic stroke and acute myocardial infarction |
| Extra-axial intracranial neoplasm |
| Extracranial cervical dissections |
| History of ≤ 10 cerebral microbleeds on magnetic resonance imaging |
| History of myocardial infarction in past three months (if not an ST-segment elevation myocardial infarction, right, inferior, or left anterior myocardium) |
| History of warfarin (Coumadin) use and an international normalized ratio ≤ 1.7 or a prothrombin time < 15 seconds |
| Initial glucose levels of 50 to 400 mg per dL (2.77 to 22.20 mmol per L; can be treated if still indicated after correction) |
| Known unruptured and unsecured intracranial aneurysm < 10 mm |
| Mild but disabling stroke symptoms |
| Noncontrast computed tomography findings of mild to moderate early ischemic changes |
| Normal activated partial thromboplastin time and end-stage renal disease being treated with hemodialysis |
| Sickle cell disease |
| Additional considerations if patient presents within three to four and a half hours of last known well |
| Severe stroke symptoms (National Institutes of Health Stroke Scale score > 25), benefit is uncertain |
| Careful consideration, evaluate risks vs. benefits |
| Acute pericarditis (recommend consulting cardiology) |
| History of > 10 cerebral microbleeds on magnetic resonance imaging |
| History of bleeding diathesis or coagulopathy |
| History of diabetic hemorrhagic retinopathy or other hemorrhagic ophthalmic conditions |
| History of gastrointestinal or genitourinary bleeding |
| Lumbar puncture in past seven days |
| Major surgery in past 14 days |
| Pregnant patients with moderate or severe stroke |
| Recent major trauma not involving the head in past 14 days |
| Recent vaginal bleeding causing clinically significant anemia (recommend consulting gynecology) |
| Contraindications |
| Computed tomography of the brain exhibits extensive regions of clear hypoattenuation |
| Computed tomography reveals acute intracranial hemorrhage |
| Gastrointestinal bleeding event in past 21 days |
| History of intracranial hemorrhage |
| Intra-axial intracranial neoplasm |
| Intracranial or spinal surgery within past three months |
| Ischemic stroke within past three months |
| Known or suspected aortic arch dissection |
| Mild nondisabling stroke (National Institutes of Health Stroke Scale score of 0 to 5) |
| Patients taking direct thrombin inhibitors or direct factor Xa inhibitors in past 48 hours |
| Patients who have received a full treatment dose of low-molecular-weight heparin within the past 24 hours |
| Platelet count < 100,000 per mm3 (100 x 109 per L), international normalized ratio > 1.7, activated partial thromboplastin time > 40 seconds, or prothrombin time > 15 seconds |
| Severe head trauma within past three months |
| Signs and symptoms concerning for subarachnoid hemorrhage |
| Symptoms concerning for infective endocarditis |
| Unknown usefulness and risk |
| Current malignancy |
| Known or suspected intracranial arterial dissection |
Subarachnoid hemorrhage should be considered in patients with sudden onset of a severe atraumatic headache that is described as the worst headache of their life.19 A lumbar puncture should be considered for further evaluation, especially in patients who present six hours after headache onset, because subarachnoid hemorrhage can be missed by NCCT and MRI. The Ottawa Subarachnoid Hemorrhage Rule for Headache Evaluation can be used to rule out subarachnoid hemorrhage with 100% sensitivity in patients 15 years and older who present with new severe atraumatic headache with maximum intensity within one hour. With any positive findings (i.e., age 40 years and older; neck pain or stiffness; witnessed loss of consciousness; onset during exertion; thunderclap headache [pain peaking within one second]; and limited neck flexion), subarachnoid hemorrhage cannot be ruled out.19–21
In patients with cerebellar symptoms that are concerning for posterior circulation stroke (Table 65), such as gait ataxia, limb ataxia, or vertigo, a HINTS (head-impulse, nystagmus, test of skew) examination should be performed to determine a CNS vs. peripheral cause. It is more sensitive for stroke than early MRI, with a sensitivity of 100% and a specificity of 96%.22–24 A diffusion-weighted MRI is the preferred imaging study, but a negative early MRI may need to be repeated in three to seven days or followed up with a HINTS examination5,17,22 (Table 75,22–25). A video demonstrating the HINTS examination is available at https://www.youtube.com/watch?v=1q-VTKPweuk.
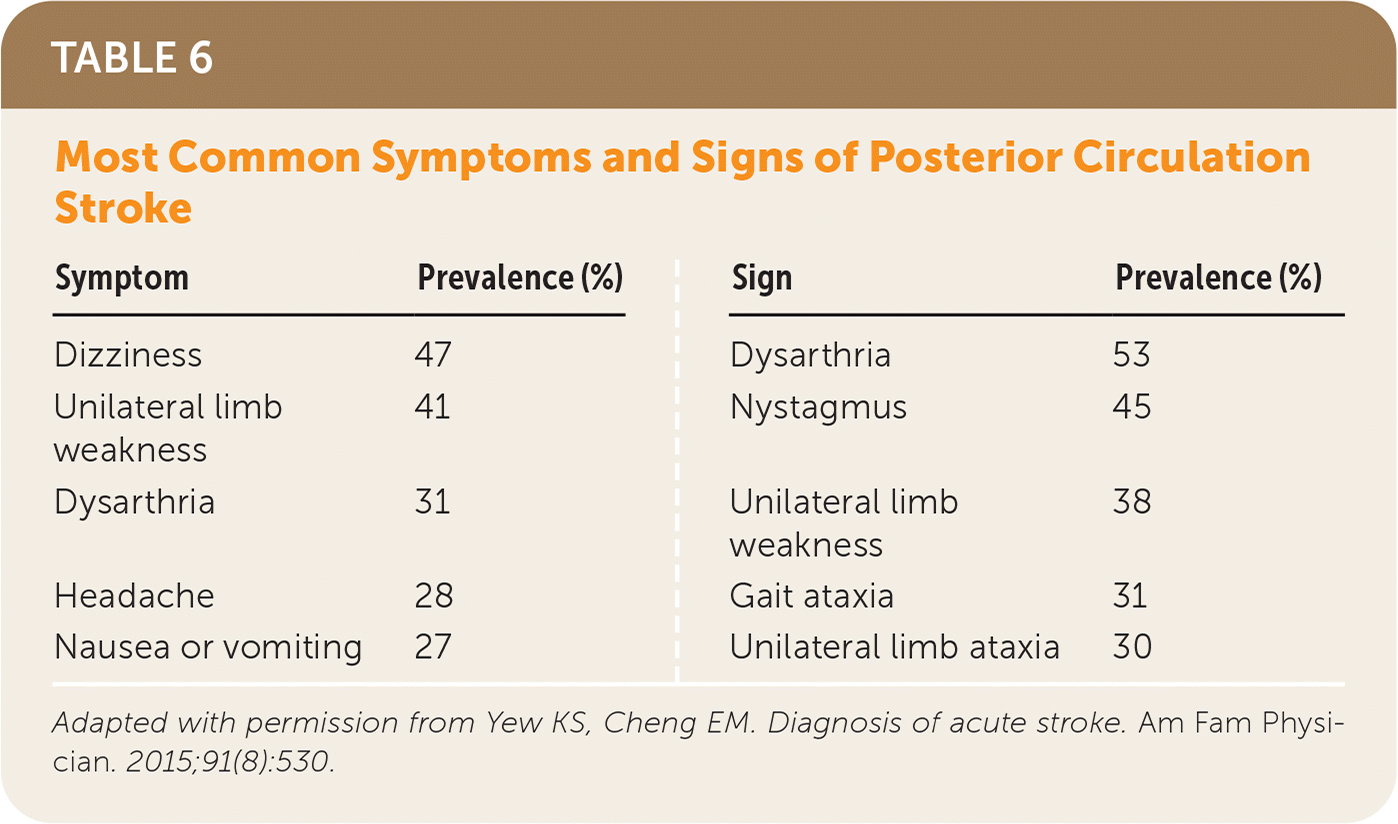
| Symptom | Prevalence (%) | Sign | Prevalence (%) |
|---|---|---|---|
| Dizziness | 47 | Dysarthria | 53 |
| Unilateral limb weakness | 41 | Nystagmus | 45 |
| Dysarthria | 31 | Unilateral limb weakness | 38 |
| Headache | 28 | Gait ataxia | 31 |
| Nausea or vomiting | 27 | Unilateral limb ataxia | 30 |

| Head-impulse test | The patient should focus on a fixed target. Turn patient's head laterally 10 to 20 degrees with high acceleration while observing the eyes. A saccadic eye movement is indicative of a peripheral cause. |
| Nystagmus test | The patient should look with a sustained gaze in a left or right direction. Do not have the patient fixate on a target. The fast beat of the nystagmus that changes direction with change ingaze (refixation), vertical nystagmus, or torsional nystagmus is consistent with a central nervous system pathology. |
| Test of skew | The patient should focus on a fixed target, typically the examiner's nose. Perform cover-uncover test on each eye. Any upward or downward gaze correction is indicative of central nervous system pathology. |
Large Vessel Occlusion
Recent data from multiple randomized trials have shown that patients with large vessel occlusion have significantly better outcomes with endovascular treatment.9,18,26–28 Patients presenting within 24 hours with severe neurologic deficits thought to be caused by large vessel occlusions of the internal carotid arteries or proximal middle cerebral arteries should undergo noninvasive cerebrovascular imaging with computed tomographic angiography (CTA) or magnetic resonance angiography (MRA). This allows for the evaluation of ischemia and large vessel occlusion and assessment of the ratio of potentially reversible ischemic tissue to the volume of infarcted tissue to determine eligibility for mechanical thrombectomy.9,18,29 Evaluation with noninvasive imaging has a sensitivity of 87% to 100% and specificity of more than 95% for detecting large vessel occlusions, with CTA having greater accuracy than MRA.8,9 Magnetic resonance transcranial Doppler ultrasonography is less sensitive than angiography (46% to 60%) but can inform improved treatment outcomes of rtPA therapy.8,9
Digital subtraction angiography (conventional angiography) is the diagnostic standard for determining large vessel involvement.8 Neurologic complications, including stroke, increase with invasive imaging, but based on a large prospective study the risk is about 1%.30 Intra-arterial treatments include mechanical thrombectomy, which is performed with a stent retriever or an aspiration catheter, or administration of intra-arterial fibrinolysis. Both approaches may be used in combination. The success of mechanical clot removal depends on determining the length of the clot.
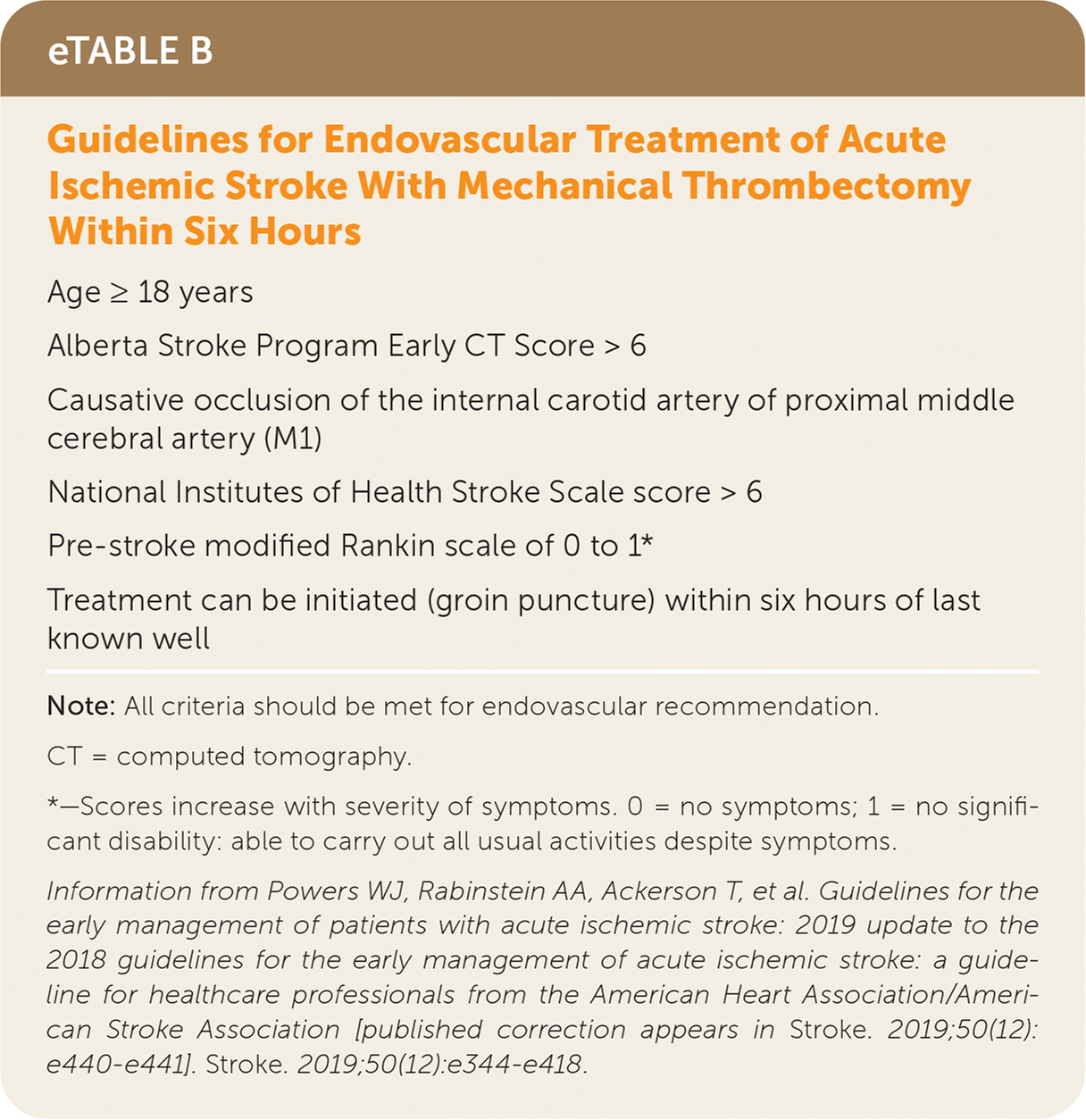
| Age ≥ 18 years |
| Alberta Stroke Program Early CT Score > 6 |
| Causative occlusion of the internal carotid artery of proximal middle cerebral artery (M1) |
| National Institutes of Health Stroke Scale score > 6 |
| Pre-stroke modified Rankin scale of 0 to 1* |
| Treatment can be initiated (groin puncture) within six hours of last known well |
Poststroke Evaluation
When the initial evaluation is completed, whether or not the patient received rtPA therapy, a full evaluation is necessary to determine the etiology of the stroke. If rtPA was administered, diffusion-weighted MRI should be performed before starting anticoagulants or antiplatelet agents. CTA or MRA is an appropriate imaging technique to evaluate for carotid artery stenosis, but digital subtraction angiography is the diagnostic standard.8 Carotid Doppler ultrasonography has less sensitivity (83% to 86%) than any radiologic evaluation. CTA has more than 90% sensitivity for carotid artery stenosis. Echocardiography should be performed to evaluate for a cardiac source of embolic strokes. In patients 18 to 60 years of age, echocardiography with bubble study should be used to determine if there is an anomalous opening between the right and left side of the heart, such as a patent foramen ovale, for consideration of closure of the abnormality. The RESPECT trial showed a significant reduction in recurrent ischemic strokes with patent foramen ovale closures.32
Transesophageal echocardiography is more sensitive than transthoracic echocardiography, but it is also more invasive. Transesophageal echocardiography should be performed in patients who have a high probability of a cardiac source of embolism, are younger than 45 years without cardiac disease, have mechanical valves, or have suspected aortic pathology.33 All patients with stroke should be placed on telemetry while in the hospital to evaluate for paroxysmal atrial fibrillation. Two studies have shown that 10% to 15% of patients with a diagnosis of stroke, not otherwise specified without atrial fibrillation who were on 24 hours of telemetry were diagnosed with paroxysmal atrial fibrillation on a 30-day loop recorder in the following six months after their stroke, further supporting the need for long-term monitoring for arrhythmia in older, high-risk individuals following unspecified strokes.34,35
This article updates previous articles on this topic by Yew and Cheng5 and Yew and Cheng.36
Data Sources: A PubMed search was completed in Clinical Queries using the key terms stroke, stroke evaluation, AAN guidelines, stroke imaging, post stroke care, stroke subtypes, stroke differential, and treatment. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. We also used search results from the Cochrane database, Essential Evidence Plus, UpToDate, and Dynamed. Search dates: March 15, 2021, and April 10, 2022.
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or reflecting the views of the Department of the Army, Department of Defense, or the U.S. government.
