
Am Fam Physician. 2022;105(6):665-666
Author disclosure: No relevant financial relationships.

| Test | Indication | Population and frequency | Cost* |
|---|---|---|---|
| Multiparametric magnetic resonance imaging | Risk stratification for targeted biopsy; active surveillance of low-risk prostate cancer | Patients 50 years or older with suspected or known prostate cancer, frequency of test varies | $275 to $444 |
Accuracy
The prostate imaging reporting and data system, second revision (PI-RADSv2) provides standardized interpretation and reporting of mpMRI.1 A score between 1 and 5 is assigned by a radiologist (1 suggests that a clinically significant cancer is highly unlikely, and 5 suggests that it is highly likely). In a systematic review of 21 studies including 3,857 patients, the PI-RADSv2 tool (using a threshold score of 3 or 4) had a pooled sensitivity of 0.89 (95% CI, 0.86 to 0.92) and specificity of 0.73 (95% CI, 0.60 to 0.83) for the detection of prostate cancer.4
Inclusion of mpMRI information into a multivariate risk-prediction calculator improves the accuracy of cancer risk assessment and can assist in shared decision-making regarding management options.5
Benefit
In a randomized controlled trial of 1,532 patients with prostate-specific antigen levels of 3 ng per mL (3 mcg per L) or greater, clinically significant prostate cancer was diagnosed in a similar percentage of patients who had mpMRI plus targeted biopsy as those who had TRUS-guided systematic biopsy alone (21% vs. 18%; risk difference = 3%; 95% CI, −1% to 7%). Additionally, clinically insignificant cancer was detected less often in the mpMRI plus targeted biopsy group compared with the TRUS-guided systematic biopsy group (4% vs. 12%; risk difference = −8%; 95% CI, −11% to −5%).7
A prospective cohort of 172 patients in whom cancer was suspected despite previous negative biopsies underwent mpMRI plus targeted biopsy and TRUS-guided systematic biopsy. Targeted biopsy detected clinically significant prostate cancer (Gleason score of 7 or more) more often than systematic biopsy (16% vs. 9%; P = .01).8
Harms
In 2017, the U.S. Food and Drug Administration started requiring a warning with gadolinium-based contrast agents because they may be partially retained in brain tissue for months to years after use.10 The only established complication of gadolinium-based contrast is nephrogenic systemic fibrosis, which affected up to 0.07% of patients with stage 4 or 5 chronic kidney disease in one large meta-analysis.11 The long-term consequences of gadolinium retention are otherwise unknown and require further safety studies.
Cost
Studies suggest that mpMRI could be cost-effective at $23,483 per quality-adjusted life-year, although this conclusion depends on the assumption that a negative mpMRI result could be used to safely avoid biopsy.12
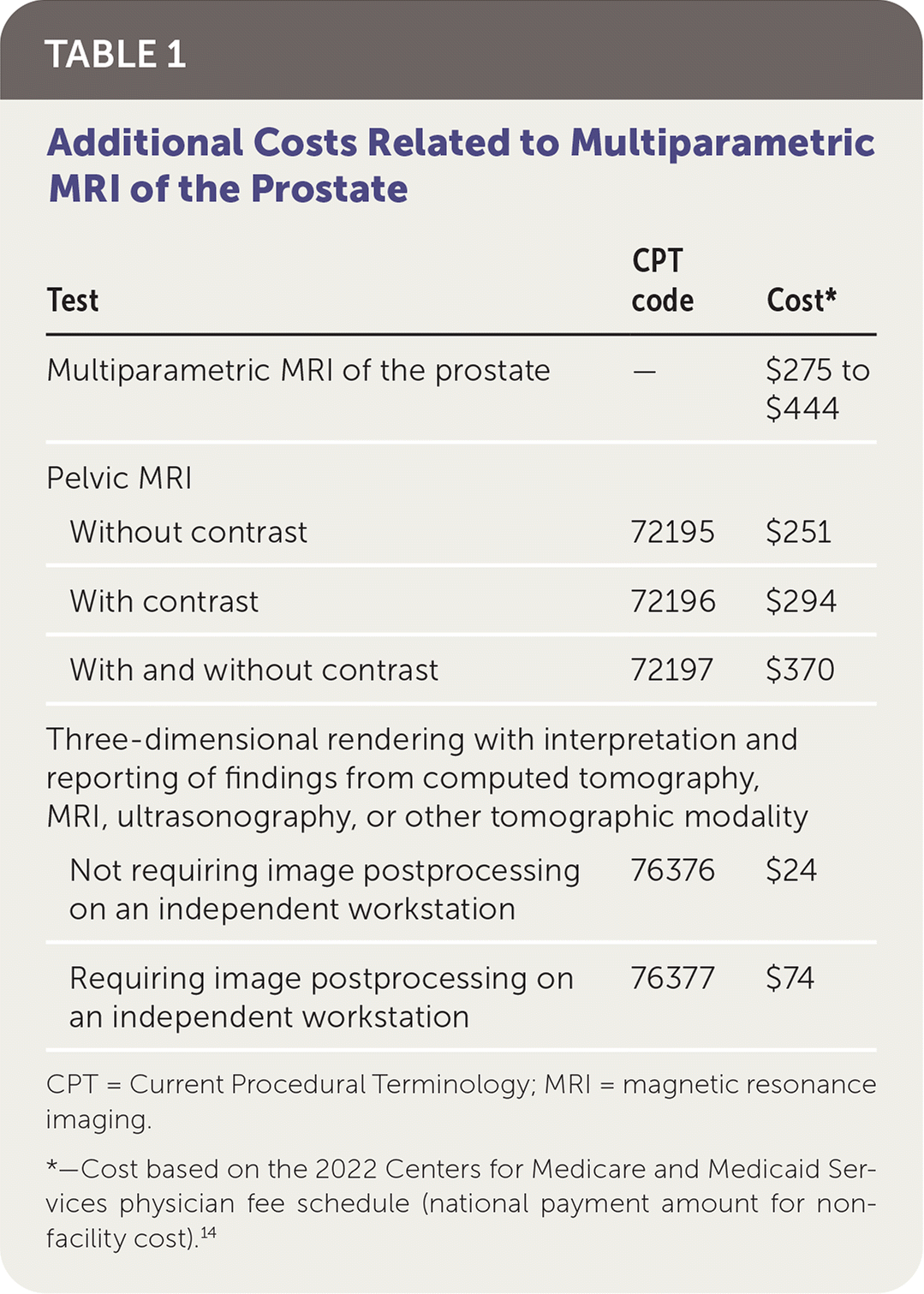
| Test | CPT code | Cost* |
|---|---|---|
| Multiparametric MRI of the prostate | — | $275 to $444 |
| Pelvic MRI | ||
| Without contrast | 72195 | $251 |
| With contrast | 72196 | $294 |
| With and without contrast | 72197 | $370 |
| Three-dimensional rendering with interpretation and reporting of findings from computed tomography, MRI, ultrasonography, or other tomographic modality | ||
| Not requiring image postprocessing on an independent workstation | 76376 | $24 |
| Requiring image postprocessing on an independent workstation | 76377 | $74 |
Bottom Line
In patients with known or suspected prostate cancer, mpMRI may improve care by providing an individualized assessment of clinically significant cancer risk and by improving the yield of prostate biopsy. However, no studies have evaluated the effects of mpMRI use on morbidity or mortality.
