
Am Fam Physician. 2022;105(6):678-679
Author disclosure: No relevant financial relationships.
Key Points for Practice
• Fidaxomicin reduces recurrence compared with vancomycin for initial and recurrent CDI.
• For patients with recurrent CDI within six months, adding bezlotoxumab to antibiotics reduces further CDI recurrence.
• Fecal microbiota transplant risks transmitting potentially fatal infections, including E. coli and SARS-CoV-2.
From the AFP Editors
Fidaxomicin Is Preferred
Although fidaxomicin (Dificid) leads to similar initial cure rates as vancomycin for CDI, fidaxomicin reduces recurrent infections. Treating an initial CDI with fidaxomicin instead of vancomycin prevents one additional recurrence at four weeks for every 10 patients treated (number needed to treat [NNT] = 10; 95% CI, 7 to 18). Treating recurrent CDI with fidaxomicin reduces recurrence at 30 days with an NNT of 7 (95% CI, 4 to 30) and at 90 days with an NNT of 5 (95% CI, 3 to 112) compared with vancomycin.
Vancomycin and fidaxomicin are oral medications with minimal systemic absorption. The twice-daily dosing of fidaxomicin is less cumbersome than the four times daily dosing required for vancomycin treatment. Resistance to fidaxomicin has rarely been reported in C. difficile. Fidaxomicin is expensive, with a cost of about $4,300 for a 10-day course compared with $75 for a 10-day course of vancomycin.
Bezlotoxumab Reduces Further Recurrence
Bezlotoxumab (Zinplava) is a monoclonal antibody against C. difficile toxin B approved by the U.S. Food and Drug Administration (FDA) for prevention of recurrent CDI in high-risk adults. Bezlotoxumab is administered as a onetime intravenous infusion of 10 mg per kg over 60 minutes.
Treatment Options for Multiple Recurrences
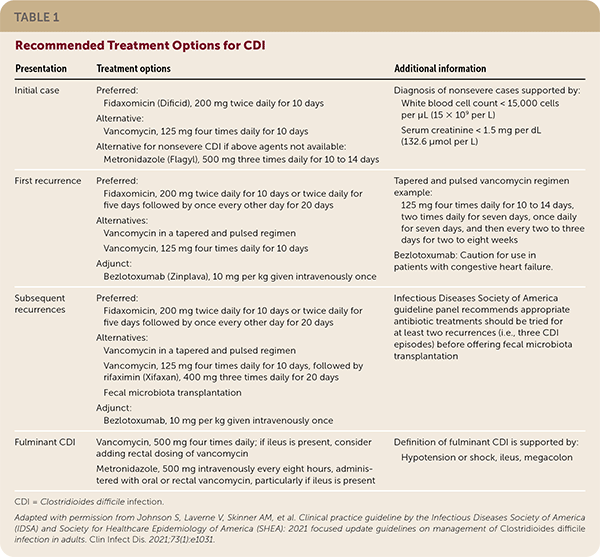
| Presentation | Treatment options | Additional information |
|---|---|---|
| Initial case | Preferred: Fidaxomicin (Dificid), 200 mg twice daily for 10 days Alternative: Vancomycin, 125 mg four times daily for 10 days Alternative for nonsevere CDI if above agents not available: Metronidazole (Flagyl), 500 mg three times daily for 10 to 14 days | Fidaxomicin: Caution for use in patients with congestive heart failure Diagnosis of nonsevere cases supported by: White blood cell count < 15,000 cells per μL (15 × 109 per L) Serum creatinine < 1.5 mg per dL (132.6 μmol per L) |
| First recurrence | Preferred: Fidaxomicin, 200 mg twice daily for 10 days or twice daily for five days followed by once every other day for 20 days Alternatives: Vancomycin in a tapered and pulsed regimen Vancomycin, 125 mg four times daily for 10 days Adjunct: Bezlotoxumab (Zinplava), 10 mg per kg given intravenously once | Tapered and pulsed vancomycin regimen example: 125 mg four times daily for 10 to 14 days, two times daily for seven days, once daily for seven days, and then every two to three days for two to eight weeks |
| Subsequent recurrences | Preferred: Fidaxomicin, 200 mg twice daily for 10 days or twice daily for five days followed by once every other day for 20 days Alternatives: Vancomycin in a tapered and pulsed regimen Vancomycin, 125 mg four times daily for 10 days, followed by rifaximin (Xifaxan), 400 mg three times daily for 20 days Fecal microbiota transplantation Adjunct: Bezlotoxumab, 10 mg per kg given intravenously once | Infectious Diseases Society of America guideline panel recommends appropriate antibiotic treatments should be tried for at least two recurrences (i.e., three CDI episodes) before offering fecal microbiota transplantation |
| Fulminant CDI | Vancomycin, 500 mg four times daily; if ileus is present, consider adding rectal dosing of vancomycin Metronidazole, 500 mg intravenously every eight hours, administered with oral or rectal vancomycin, particularly if ileus is present | Definition of fulminant CDI is supported by: Hypotension or shock, ileus, megacolon |
Fecal Microbiota Transplantation
Evidence suggests an increased risk from fecal microbiota transplantation (FMT) in CDI. Safety alerts from the FDA highlight the risk of transmission of pathogenic Escherichia coli and SARS-CoV-2 through FMT, both of which can be fatal. FMT can be considered after multiple recurrences of CDI despite appropriate antibiotic treatments. When FMT is performed, the FDA recommends careful screening of donors and fecal specimens.
Editor's Note: The IDSA significantly changed their recommendations for treatment of CDI in this guideline update. Based on a demonstrated reduction of CDI recurrence, fidaxomicin is now recommended over vancomycin for initial and recurrent infection. Fidaxomicin has been detailed in a previous STEPS review (https://www.aafp.org/afp/2013/0201/p211.html). Immunotherapy with bezlotoxumab with antibiotics is recommended for recurrent CDI. Risks of fatal infections have reduced the acceptability of fecal microbiota transplantation. These recommendations are newer than those in the latest AFP article on CDI (https://www.aafp.org/afp/2020/0201/p168.html), which was based on previous guidelines. The newly recommended treatments add significant cost to the management of CDI.— Michael J. Arnold, MD, Contributing Editor
Dr. Finke is the AFP medical editing fellow. The author calculated the NNTs using data provided in the guideline.
Guideline source: Infectious Diseases Society of America
Evidence rating system used? Yes
Systematic literature search described? Yes
Guideline developed by participants without relevant financial ties to industry? No
Recommendations based on patient-oriented outcomes? Yes
Published source: Clin Infect Dis. September 7, 2021;73(5):e1029–e1044
