
Am Fam Physician. 1999;60(6):1727-1734
The principal cause of right ventricular infarction is atherosclerotic proximal occlusion of the right coronary artery. Proximal occlusion of this artery leads to electrocardiographically identifiable right-heart ischemia and an increased risk of death in the presence of acute inferior infarction. Clinical recognition begins with the ventricular electrocardiographic manifestations: inferior left ventricular ischemia (ST segment elevation in leads II, III and aVF), with or without accompanying abnormal Q waves and right ventricular ischemia (ST segment elevation in right chest leads V3R through V6R and ST segment depression in anterior leads V2 through V4). Associated findings may include atrial infarction (PR segment displacement, elevation or depression in leads II, III and aVF), symptomatic sinus bradycardia, atrioventricular node block and atrial fibrillation. Hemodynamic effects of right ventricular dysfunction may include failure of the right ventricle to pump sufficient blood through the pulmonary circuit to the left ventricle, with consequent systemic hypotension. Management is directed toward recognition of right ventricular infarction, reperfusion, volume loading, rate and rhythm control, and inotropic support.
Clinicians sometimes encounter a patient who presents to the hospital with signs and symptoms of an acute myocardial infarction and is discovered, based on large ST segment elevations in leads II, III and aVF on the electrocardiogram (ECG), to be having an acute inferior myocardial infarction. In this situation, clinicians first need to determine if the occlusion is strictly a left ventricular (LV) infarction or if it is also jeopardizing the right ventricular (RV) free wall and septum. RV injury increases the risk of mortality in the elderly. Its recognition requires volume loading and rate and rhythm control, measures that otherwise are not part of the usual treatment of myocardial infarction.
Recognition and Diagnosis of Right Ventricular Infarction
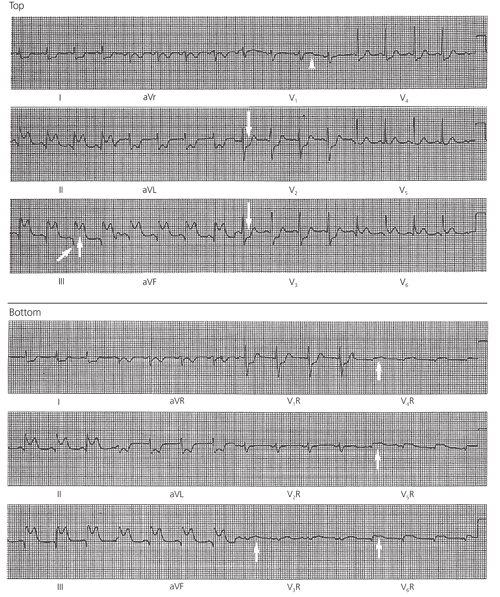
Approximately one half of patients who present with signs and symptoms of acute inferior myocardial infarction have proximal occlusion of the dominant right coronary artery (RCA) and also show ECG signs of RV wall ischemia or infarction (Figure 1). Table 1 groups the branches of the four major segments of the RCA in descending order, along with the corresponding regions of perfusion and the ECG findings when these regions are underperfused. Occlusion sufficiently proximal to cause RV free wall injury also frequently compromises the blood supply to the sinoatrial node, atrium and atrioventricular (AV) node, producing such effects as sinus bradycardia, atrial infarction, atrial fibrillation and AV block (Figures 2 and 3).
| Arterial segment | Arterial branch | Perfused region | ECG effects of ischemia |
|---|---|---|---|
| Proximal segment | SA nodal branch | SA node | Sinus bradycardia |
| Right atrial branch | Atrial free wall | Atrial infarct pattern, atrial fibrillation | |
| Middle segment | Lateral RV branches | Lateral RV free wall | ST segment elevation; later, abnormal Q waves in leads V3R through V6R |
| Marginal RV branch | Inferior (posterior) RV free wall | ||
| Distal segment | AV nodal branch | AV node | AV block |
| Posterior descending segment | Posterior lateral LV branches Posterior descending artery | Posterior left ventricle Inferior septum, inferior LV free wall | ST segment elevation; later, abnormal Q waves in leads II, III and aVF |
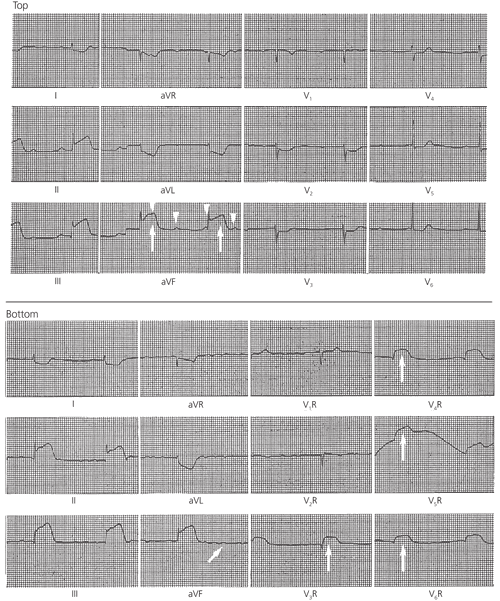
Key ECG findings occur with RV and LV ischemic wall injuries. For injury of the LV wall, these findings are ST segment elevations and, possibly, abnormal Q waves in leads II, III and aVF. The appearance of abnormal Q waves (new, wider than previously seen or wider than 0.04 seconds) may be delayed several hours; indeed, if thrombolysis or angioplasty is used acutely, the development of Q waves may be completely aborted.1–4
When involvement of the RV free wall is suspected, clinicians are alerted by the presence of relative ST segment depression in lead V2 or V3 compared with lead V1(Figure 1, top). If the ST segment depression in lead V2 is more than one half the amplitude of the ST segment elevation in lead aVF, the likely diagnosis is inferoposterior LV infarction with “reciprocal” ST segment depression in lead V2 but no RV involvement.5 However, confirmation of RV ischemia (or the clinical syndrome of RV stun or infarction) can be quickly obtained when right-sided leads V4R through V6R show ST segment elevations greater than 1 mm, or 0.1 mV (Figure 1, bottom). (The sites for right-sided leads are the mirror image of those for the usual left-sided leads: in the fifth right intercostal space, lead V4R is at the midclavicular line, lead V5R is at the anterior axillary line and lead V6R is at the midaxillary line.)
ECG identification of ischemic dysfunction is sufficient for immediate diagnosis and treatment.2 Most acute “RV infarctions” diagnosed by right-sided ST segment elevations do not progress to myocardial necrosis and subsequent scar formation. This accounts for the far lower percentage of autopsy-reported RV infarcts than clinically suspected RV infarcts.6–9 The latter group includes many patients with a stunned or hibernating RV free wall, which recovers more readily than a similarly injured LV wall. This more ready recovery occurs in part because of the rich collateral perfusion of the RV free wall and septum from the left coronary artery and the relatively greater penetration from the blood cavity by the thebesian veins. The work demand for the lower-pressure right circuit is also relatively less than that for the left circuit.
Although “RV infarction” sometimes is a misnomer, by current convention the term identifies the syndrome of acute ischemic RV dysfunction (Table 2). This syndrome is a significant clinical entity with a special pathophysiology and a well-delineated set of priorities for management.
| Right-sided (leads V3R through V6R) ST segment elevation of greater than 1 mm |
| Right ventricular asynergy as demonstrated by echocardiography or cardiac nuclear imaging |
| Mean right arterial pressure of 10 mm Hg or greater, or a less than 5 mm Hg difference from mean pulmonary capillary wedge pressure (equivalent to left atrial pressure) |
| Noncompliant right atrial pressure waveform pattern (steep and deep x and y descents) |
With RV infarction, the ECG may show an acute anterior Q-wave pattern (leads V1 through V3) as well as a right-sided Q pattern (leads V3R through V6R). A number of case reports have described this pattern in association with known occlusion of a ventricular branch of the RCA following proximal angioplasty.10,11 RV involvement with physiologic stun and electrical shutdown may well include the RV portion of the interventricular septum and thus remove much of the septal contribution to the anteroseptal and right precordial R wave.
Clinicians should recognize that in the evolving picture of RV infarction, the appearance of Q waves in leads V1 through V3 does not necessarily require changing the clinical diagnosis to anteroseptal LV infarction. The patient may well have predominant RCA occlusion and RV infarction. Therefore, the clinical alert for the hemodynamic effects of RV infarction should remain in force.
Prognosis
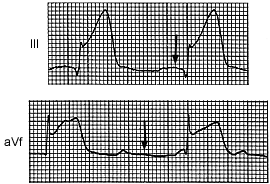
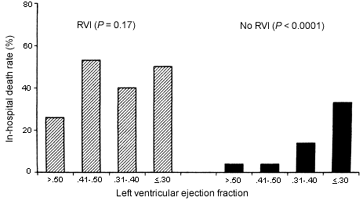
RV involvement alters the in-hospital prognosis radically by overriding the linear relationship between survival and LV ejection fraction (which, in turn, relates inversely to LV infarct size).12 Not only does the paralyzed right heart fail to offer sufficient preload to the left ventricle, but proximal occlusion of the RCA sabotages the normal regular rate and rhythm controls to the whole heart, so that cardiogenic shock remains the primary immediate cause of death, especially in the elderly. To this is added the risk of ischemic rupture of the ventricular septum with RV infarct (Figure 4).12
Yet patients who survive the hospital period enjoy the relatively good long-term prognosis of patients with inferior infarction.6 A few such patients may later develop postinfarction angina, and other patients with proximal RCA stenosis without known RV infarction may also have chronic angina pectoris. Recognition of postinfarction ongoing RV ischemia may be accomplished using either a stress test with right-sided leads or a dobutamine (Dobutrex) echocardiographic stress test. These tests will show the signs of RV ischemic dysfunction, ST segment elevations in lead V4R or RV asynergy.13
Treatment Differing from That for Left Ventricular Infarction
VOLUME LOADING
Approximately one third to one half of patients with acute inferior LV infarction and accompanying RV infarction show the effects of LV volume underload (Table 3).14–16 For many years, physiologic experiments on the canine heart portrayed the right ventricle as a passive conduit for returning venous blood to the pulmonary circuit and on to the left atrium and ventricle. In humans it has become obvious that the pumping contribution of the right ventricle is significant. Acute underperfusion of the RV free wall and adjacent interventricular septum leads to a stunned, noncompliant RV myocardium.
| Systolic RV dysfunction ⇒ | |
| ↓ RV CO ⇒ ↓ LV CO | |
| RV dilatation | |
| ↑ RV volume ⇒ septal shift | |
| Diastolic RV dysfunction ⇒ ↓ compliance ⇒ | |
| ↑ RVDP ⇒ septal shift ⇒ ↓ LVDP ⇒ ↓ LV CO | |
| ↑ RAP ⇒ ↓ septal shift ⇒ ↓ LAP ⇒ ↓ LV CO | |
Asynergy of the RV free wall (especially posteriorly and laterally) may be seen on echocardiography. Depending on the degree of ischemic damage, the hemodynamic effects may include elevated jugular venous pressure, a positive Kussmaul's sign (paradoxically increased jugular pressure or distention on inspiration) and a noncompliant pattern of the right atrial pulse waveform similar to that of constrictive pericarditis.
Loss of RV contractility may lead to a serious deficit in LV preload with a resultant drop in cardiac output and consequent systemic hypotension—an undesirable complication in the presence of acute myocardial infarction. In contrast to the low output and congestive heart failure resulting from extensive LV infarction without RV involvement, which mandates parsimonious fluid administration, the volume-underload state in RV stun may require vigorous fluid administration.
REPERFUSION
A series of meticulous experimental occlusions of the canine RCA has shown that the response to reperfusion depends on the duration of the preceding ischemia.17,18 Early reperfusion leads to prompt improvement and subsequent recovery of RV free wall contraction and global RV function without any scar formation. Late reperfusion produces little acute return of RV free wall contractile function. Because resumed perfusion increases wall thickness, septal and free wall dyskinesis is reduced, and cavitary volume is diminished. These factors permit LV contraction to pull the passive right ventricle inward, and global function then improves. A further benefit is that necrosis and subsequent scar formation are minimized.16
The advantages of the right ventricle over the left ventricle in sustaining ischemic insult are listed in Table 4. These become reasons for extending the time window for assuming reversibility when RV infarction complicates inferior LV infarction. The other reasons are that in-hospital mortality and complication rates go up with RV infarction, thereby removing the traditional, more benign connotation from inferior myocardial infarction. Stepwise management of right ventricular infarction is presented in Table 5. (Additional information on terminology and dosages is also available.19)
| Variable | Right ventricle | Left ventricle |
|---|---|---|
| Systolic pressure | Lower | Higher |
| Wall stress | Lower | Higher |
| Oxygen consumption | Less | More |
| Wall thickness | Thinner | Thicker |
| Subendocardial resistance to perfusion | Less | More |
| Coronary perfusion | Diastolic and systolic | Diastolic only |
| Recognition | ST segment elevation in leads II, III and aVF plus ST segment elevation in leads V3R through V6R | ||
| Reperfusion | Thrombolysis: | ||
| Streptokinase (Streptase), 1.5 MU given IV over 60 minutes | |||
| or | |||
| rt-PA (recombinant alteplase [Activase]) given first in 15-mg IV bolus, then 50 mg given over 30 minutes followed by 35 mg given over 60 minutes | |||
| or | |||
| rt-PA given in 10-MU IV bolus, with 10-MU bolus given 30 minutes later | |||
| Angioplasty | |||
| Coronary bypass surgery | |||
| Volume loading | Normal saline, 40 mL per minute given IV up to total of 2 L, keeping RA pressure at less than 18 mm Hg; hemodynamic monitoring required | ||
| Inotropic support | Dobutamine (Dobutrex), 2 to 5 μg per kg per minute given IV, with dose increased every 5 to 10 minutes up to 15 to 20 μg per kg per minute | ||
| Rate and rhythm control | Symptomatic bradycardia: atropine, 0.5 to 1 mg given IV every 5 minutes up to total of 2.5 mg | ||
| AV block: AV sequential pacing (usually short term) | |||
| Complications | LV ischemic dysfunction: judicious afterload reduction (managed with angiotensin-converting enzyme inhibitors); volume restriction | ||
| Cardiogenic shock: aortic balloon pump | |||
| Interventricular septal rupture: emergency surgical repair | |||
| RV papillary muscle rupture and tricuspid regurgitation: emergency surgical repair | |||
INOTROPIC SUPPORT AND RATE AND RHYTHM CONTROL
Proximal occlusion of the RCA (Table 1) not only endangers right-sided atrial and ventricular pumping functions but also rate and conduction controls. If the response to fluid loading is not adequate, a trial of intravenous dobutamine is reasonable to increase contraction strength. The response should be confirmed by bedside echocardiography and cardiac output monitoring.20
Remembering the need for atrial contribution and the vulnerability of the hampered RV output to a slow rate, clinicians may turn to temporary AV pacing for symptomatic bradycardia (whether from sinus bradycardia or third-degree AV block). It is important to maintain AV synchrony from the outset. Thus, temporary AV sequential pacing should be considered in certain patients with RV infarction to provide the needed ventricular output for the fragile period of the first three or four hospital days.20 Later, permanent pacing may be indicated.21