
Am Fam Physician. 2000;61(8):2419-2426
See related patient information handouts on asthma and asthma medications, adapted by the authors of this article.
The treatment of asthma, according to current guidelines, requires complex treatment regimens that change as clinical conditions improve or deteriorate. We have developed a practical way to communicate long-term treatment plans in chart form in the primary care setting that is easy for patients to follow and use. The chart has been an important element in two interventions that have resulted in positive changes in health behavior and health outcomes in children with asthma. The plan provides recommendations for patients and families to make adjustments in medication based on changes in symptoms or peak expiratory air flow, or both, that are consistent with the Asthma Guidelines Expert Panel Report 2, 1997. The plan also indicates when the number and dosage of drugs should be increased or decreased and when emergency care should be sought, consistent with the Asthma Guidelines. By placing considerable control in the family's hands and by clearly delineating the conditions under which medicines can be reduced or discontinued, the physician provides incentives for families to adhere to the long-term treatment plan for asthma.
It is recognized that 40 to 50 percent of prescriptions are not followed correctly and that physicians cannot accurately predict who will adhere to the therapeutic regimen.1 Effective communication with patients is likely to improve their adherence to drug regimens.1–4 In this article, we emphasize the importance of developing a long-term treatment plan for asthma that involves the patient and the family so that they will agree to follow it, and we describe a practical way to communicate such a plan, using charts to make it easier to understand and use. We have used this format in two separate, randomized controlled interventions that resulted in significant improvement in the health of children with asthma.5,6 While our research involving use of this format has been limited to children, we believe that it would also benefit adults. The format enables the physician to provide guidance to the patient so that the therapeutic regimen can be altered when circumstances change. Pharmacologic regimens that are selected by the clinicians and are consistent with the National Asthma Education and Prevention Program's guidelines recommended in the Expert Panel Report 27 can be put into this format.
Importance of a Written Plan for Complex Therapeutic Regimens
Treatment of asthma that is of greater severity than “mild intermittent,” as defined by current guidelines,7 involves the use of medications for quick relief and long-term control. Medications for long-term control are prescribed on a continuous basis in order to prevent or suppress any underlying chronic airway inflammation. Given the reluctance of many patients (including their parents) to agree to continuous use of medications, and because it is difficult to manage several medicines at once, a long-term plan needs to be developed. It should be easy to follow, be consistent with the patient's personal goals and daily activities, outline the circumstances that will lower the requirement for medication and be one that the patient and family agree to follow once the risks and benefits are discussed.
Without specific recommendations, patients often adjust or stop medications on their own. The treatment algorithms published by the expert panel on asthma7 are medically sound, but it can be difficult to adapt them to written instructions that are easy for patients and families to follow. Three elements of the clinician's encounter with patients have been identified as important in the effective management of disease by patients.8 These are (1) communicating with the patient and family in such a way as to make learning optimal; (2) providing an adequate therapeutic regimen; and (3) delivering core asthma messages, the basic information needed for patients to understand and act on the regimen prescribed. Good communication by physicians in a recent study6 led to shorter patient visits and greater patient satisfaction when compared with a control group.
Although this article focuses on pharmacologic approaches to treatment, preventive approaches that emphasize decreasing exposure to allergens and irritants such as tobacco smoke are also very important. Patients and their families can play an important role in controlling the disease by carrying out appropriate environmental controls and by following a long-term plan for monitoring and treating asthma.
Features of an Ideal Plan
An ideal plan lays out the specific steps that patients can take under changing clinical conditions. It must also provide guidelines for when to seek urgent or emergency medical care. The plan is constructed with the patient and the family to ensure that it can be incorporated into the patient's daily activities and is consistent with patient goals (Table 1). The long-term plan facilitates patient self-regulation.9 Finally, the plan must be presented in a way that is convenient and easy to visualize (e.g., on the refrigerator door or family bulletin board).
| Fewer symptoms of asthma |
| Normal physical activity (e.g., basketball, soccer, swimming) without cough or wheeze |
| Nighttime free of cough or wheeze |
| Fewer surprise episodes |
| No adverse effects of medication and, when possible, less medicine |
| Avoidance of medical emergencies |
Since the long-term plan depends on recognizing changes in the severity of signs and symptoms or peak expiratory flow, or both, it is necessary to provide adequate instruction to assure understanding of all of the measurements being used.
Finally, we assume that (1) all metered-dose inhalers are used with spacer devices (at least early in treatment) to ensure that the medication is reaching the airways while reducing deposition in the mouth, and (2) patients and families have been taught to administer the medicines correctly and have demonstrated this competence to their clinician.
Although we will focus on the use of metered-dose inhalers, dry powder inhalers are also now available. While nebulizers are popular for the treatment of acute exacerbations and for the treatment of infants and young children, it is also possible in these circumstances to give the medication using metered-dose inhalers and spacers with a face mask if the child is cooperative. One study10 recently reviewed the evidence that supports the view that administering beta2 agonists by metered-dose inhalers with valved aerosol holding chambers (spaces) can be therapeutically equivalent to administration by nebulizer even in patients with more serious acute asthma, provided there is patient acceptability of the delivery device chosen.
Rationale for the Therapeutic Regimen
The therapeutic regimens currently recommended by the National Asthma Education and Prevention Program report are based on severity (mild-intermittent, mild-persistent, moderate-persistent and severe-persistent) as judged by the frequency of daily symptoms, nighttime symptoms and the need for short-acting bronchodilators (beta2 agonists) or by changes in peak air flow measurements.7
The four classifications are as follows: mild-intermittent asthma—symptoms occur two times a week or less and generally require a short-acting beta2 agonist (classified as relievers) only on an as-needed basis; mild-persistent asthma—symptoms occur more than twice a week but less than once a day; moderate-persistent asthma—symptoms occur daily; severe-persistent asthma—symptoms are continual.
Patients with persistent asthma require a regular controller medication as well as a reliever. However, even those with intermittent asthma can experience severe exacerbations requiring short-acting beta2 agonists every four hours. When this is required for more than 24 hours more often than every six weeks, a long-term controller medicine is recommended.7 The latter are primarily drugs that are anti-inflammatory—corticosteroids are the most effective.
Since respiratory infections are a common trigger for asthma, especially in early life,11 it may be desirable to start regular use of beta2 agonists at the earliest sign of a cold; for those who have particularly severe exacerbations of asthma with respiratory infections, early treatment with anti-inflammatory medication may also be necessary even in those with mild-intermittent asthma.
Making a Chart for the Long-Term Plan
Sample charts for organizing the clinician's recommendations7 are shown in Figures 1 through 4. The medications are listed vertically on the left side of the chart. The doses and number of times per day appear in the cells. Across the top are listed a series of common clinical circumstances. This underscores that conditions are expected to change and patients are expected to respond appropriately to change. Each column represents the medications to be taken for that clinical condition. In our experience, families like the chart, use it and often bring it with them on return visits. The chart also facilitates discussions of treatment by telephone.
The footnotes to Figures 1 through 4 provide clinicians with additional directions or options discussed in the asthma guidelines7 to deal with the current complexities of treating asthma while preserving the relative simplicity of the chart. They also contain recent recommendations with respect to antileukotrienes.12 The extensive footnotes are omitted from the charts when they are given to the patient. The information in the footnotes regarding when to consider corticosteroids and when to seek emergency care should be provided directly to the patient and the family by the physician.
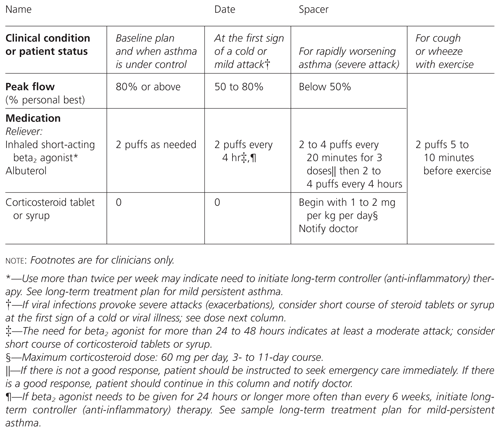


MILD-INTERMITTENT ASTHMA
The baseline recommendation plan is two puffs of a quick reliever or short-acting beta2 agonist such as albuterol on an as-needed basis (Figure 1). At the first sign of a cold, exposure to known trigger or mild attack, patients are asked to take two puffs of albuterol every four hours. For rapidly worsening asthma (severe exacerbation or attack), the recommendation is to give two to four puffs of albuterol every 20 minutes for three doses. If symptoms do not decrease, emergency care should be sought. If there is improvement, albuterol can be continued at two to four puffs every four hours, and corticosteroids (tablets or syrup) are started. We also indicate that the patient should be evaluated by the physician to determine whether another condition is present (e.g., infection) that requires treatment.
As recommended by the asthma guidelines, oral corticosteroids (prednisone or equivalent) are given for three to 11 days at an initial dosage of 1 to 2 mg per kg per day, with a maximum dosage of 60 mg per day. When asthma is under control, two puffs of albuterol are required only as needed. Finally, for cough or wheezing with exercise, the patient is asked to take two puffs of albuterol just before exercise. The corresponding levels of peak flow as percentage of personal best are also shown on this chart. Thus patients and families can use either symptoms or peak flow measurements as a basis for deciding on treatment.
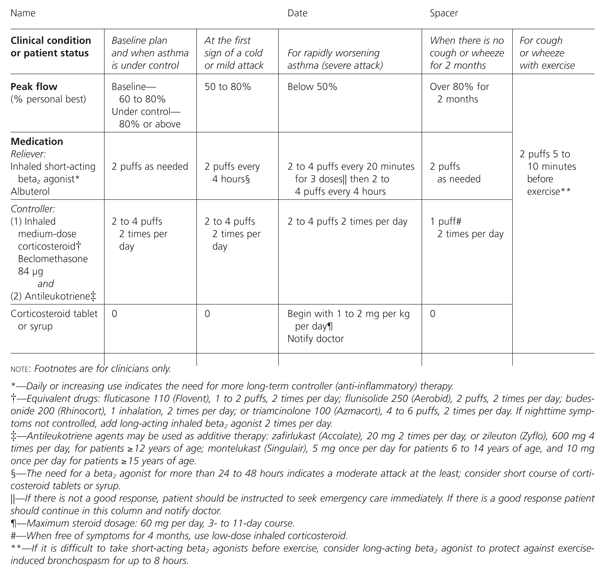
MILD-PERSISTENT ASTHMA
For cases of increased asthma severity, the chart contains an additional row for controller medication, and equivalent dosages for all the other current inhaled steroids (Figure 2) are listed in the footnotes. The asthma guidelines recommend that, for young children, non-steroid controllers be tried before steroids. The chart also contains an additional column for treatment when there is no cough or wheezing for two months (i.e., when asthma is under control) or peak flow is above 80 percent of personal best for two months. Under these circumstances, a nonsteroid controller such as nedocromil (Tilade) or cromolyn (Intal) can be used. An antileukotriene agent may be considered as an alternative.7,12
Although it is not always possible to discontinue inhaled steroids quickly, the conditions under which it would be appropriate to discontinue steroids are part of the plan. This gives families another incentive to stay on the program. We stress that staying with the plan and not discontinuing medications (especially controllers) prematurely is likely to lead to better control of asthma, less lung remodeling and less medication, especially systemic steroids, in the long term.13,14
MODERATE- OR SEVERE-PERSISTENT ASTHMA
Figure 3 provides a sample long-term treatment plan for moderate-persistent asthma. The asthma guidelines suggest that patients with moderate- or severe-persistent asthma also seek consultation with an asthma specialist in addition to their primary care physician. While we have included a sample plan for children with severe-persistent asthma (Figure 4), we think these children should be managed by family physicians only when there is also strong input from an asthma specialist. As indicated in the footnote, antileukotrienes may be used as additive controller therapy for moderate- and severe-persistent asthma.12
Figures 1 through 4 provide recommendations for treatment based on peak flow expressed as “percent personal best.” There is evidence that a symptom diary may be as useful as serial peak expiratory air flow rates in following some patients.15 The clinician should indicate whether patients should concentrate on clinical symptoms or peak flow depending on his or her assessment of the patient's or the family's ability to make the appropriate observations. We recommend that patients follow the treatment steps indicated in the charts if either symptoms or peak air flow criteria are met.
The clinician and the patient need not complete all cells of the chart during the first visit. Initially, one or two columns of the plan can be filled in to help the patient maintain control of symptoms until the next clinical visit. With further feedback from the patient about progress with the initial recommendations and the provision of further education, the chart can then be sequentially completed. In completing the chart, some compromise from an ideal regimen may be necessary to fit with the family's lifestyle, school or work schedule, cost of medication or family's concerns about potential side effects of drugs. Finally, the charts are easy to modify to accommodate new therapies.
Strategies for Enhancing Adherence
Until the physician provides some reassurance regarding the patient's underlying fears, most patients will not be able to focus on or remember the clinician's recommendations.3
LINKING TREATMENT PLANS TO PATIENT GOALS
As the physician and patient develop the long-term treatment plan together, the physician's efforts to link treatment recommendations to short- and long-term goals of the patient increases motivation to follow the plan. Patient goals of therapy that are generally shared by physicians are shown in Table 1.
EARLY AND AGGRESSIVE TREATMENT
Untreated asthma may lead to airway remodeling as well as to serious, if not life-threatening, airway obstruction. In addition, it is often difficult to get patients to follow a daily preventive treatment plan over a long period if they do not see improvement quickly. Therefore, we have adopted the strategy recommended in the Expert Panel Report 2 of treating asthma very aggressively initially, and then reducing the amount and dosage of medication as the patient improves.7 The guidelines refer to this as “stepping down.” The physician will need to assess clinical, economic and psychologic factors when deciding on the therapeutic regimen needed for each patient. In all cases, however, simplicity of the regimen and the written program presented to patients will lead to increased adherence.8
There are two major advantages to the long-term plan as communicated to patients and families in Figures 1 through 4. First, it shows families what lies ahead, what specific steps they can take and that considerable control is placed in their hands. Second, the plan provides clear criteria for when to increase and when to decrease the medication. Both the sense of control and the clear delineation of the conditions under which medicines can be reduced or discontinued increase patient motivation to accept and adhere to the clinical recommendations.