
Am Fam Physician. 2000;62(3):565-570
See related patient information handout on hyaluronic acid injections for knee osteoarthritis, written by the author of this article.
Knee osteoarthritis is a common but often difficult problem to manage in primary care. Traditional nonsurgical management, consisting of lifestyle modification, physical therapy and pharmacologic therapy (e.g., analgesics, anti-inflammatory medications), is often ineffective or leaves residual symptoms. Viscosupplementation is a newly available option for patients with symptomatic knee osteoarthritis that involves a series of intra-articular injections of hyaluronic acid. The exact mechanism of action is unclear, although increasing the viscoelasticity of the synovial fluid appears to play a role. Clinical experience and studies of the two hyaluronic acid products available, hyaluronan and hylan G-F 20, are inconclusive but seem to indicate beneficial effects with minimal adverse reactions in a significant number of patients. The exact indications for viscosupplementation are still evolving, but it currently can be considered for use in patients who have significant residual symptoms despite traditional nonpharmacologic and pharmacologic treatments. In addition, patients who are intolerant of traditional treatments (e.g., gastrointestinal problems related to anti-inflammatory medications) can be considered for these injections. Family physicians with the ability to perform intra-articular knee injections should consider them an option in patients with symptomatic knee osteoarthritis.
Symptomatic osteoarthritis of the knee joint is a common presenting problem in primary care and can be a frustrating problem for physicians and patients. Treatment options and their efficacies are often limited. Intra-articular injections of hyaluronic acid, known as viscosupplementation, are a recently available option.
Osteoarthritis is characterized by a loss of articular cartilage, which has a highly limited capacity to heal itself. Along with these cartilage changes, a reduction in the elastic and viscous properties of the synovial fluid occurs. The molecular weight and concentration of the naturally occurring hyaluronic acid decreases. Theoretically, this loss of elastoviscosity decreases the lubrication and protection of the joint tissues and is one postulated mechanism of pain production in osteoarthritis.1,2 Pharmacologic treatment generally consists of analgesics and/or non-steroidal anti-inflammatory drugs (NSAIDs). Physical therapy can be used, with exercises to maintain range of motion and strength. Intra-articular corticosteroid injections are often used for transient symptom relief. When conservative measures fail, surgical treatments limited to arthroscopic debridement, osteotomies to redistribute load and total joint replacements have been the only options until recently.
Viscosupplementation
The pathologic changes of synovial fluid hyaluronic acid, with its decreased molecular weight and concentration, led to the concept of viscosupplementation. Viscosupplementation came into clinical use in Japan and Italy in 1987, in Canada in 1992, in Europe in 1995 and in the United States in 1997.1 Two hyaluronic acid products are currently available in the United States: naturally occurring hyaluronan (Hyalgan) and synthetic hylan G-F 20 (Synvisc). Hylans are cross-linked hyaluronic acids, which gives them a higher molecular weight and increased elastoviscous properties. The higher molecular weight of hylan may make it more efficacious than hyaluronic acid because of its enhanced elastoviscous properties and its longer period of residence in the joint space (i.e., slower resorption).1,3
The exact mechanism of action of viscosupplementation is unclear. Although restoration of the elastoviscous properties of synovial fluid seems to be the most logical explanation, other mechanisms must exist. The actual period that the injected hyaluronic acid product stays within the joint space is on the order of hours to days, but the time of clinical efficacy is often on the order of months.4 Other postulated mechanisms to explain the long-lasting effect of viscosupplementation include possible anti-inflammatory and antinociceptive properties, or stimulation of in vivo hyaluronic acid synthesis by the exogenously injected hyaluronic acid.5
Clinical Studies of Hyaluronan
Multiple studies have been conducted to evaluate the efficacy of intra-articular hyaluronan injections (Table 1). Initial studies6–8 in the 1970s and 1980s demonstrated benefits for hyaluronan-injected knees. More recently, Dahlberg and colleagues,9 and Henderson and coworkers,10 in randomized, double-blind placebo-controlled trials found no benefit from intra-articular hyaluronan over placebo. Lohmander and associates11 similarly found no significant differences between overall treatment and placebo groups; however, a subgroup analysis of patients more than 60 years of age with more severe symptoms revealed beneficial effects from the hyaluronan injections.
| Study | Number of subjects | Results | |
|---|---|---|---|
| Dahlberg, et al.9 | 28 treatment | At one year follow-up, no difference in pain, function, activity or other parameters | |
| 24 control | |||
| Henderson, et al.10 | 45 treatment | Five months after last injection, no short- or long-term differences exist between groups for pain or other parameters. | |
| 46 control | |||
| Lohmander, et al.11 | 96 treatment 93 control | At 20-week follow-up, no overall difference; subgroup analysis: | |
| 60-years-and-older group with more severe symptoms consistently superior for pain, activity level and global assessment | |||
| Dougados, et al.12 | 55 treatment 55 control | At seven weeks, visual analog pain scale = −35.5 in the treatment group vs. –25.8 in the control group (P = 0.03) At one year: efficacy = 77 percent in the treatment group vs. 54 percent in the control group (P = 0.01) | |
| Puhl, et al.13 | 95 treatment 100 control | Lequesne Index scores decreased 4.4 points in the treatment group vs. 2.8 points in the control group (P < 0.05) At 14 weeks, visual analog pain scale = −27.6 in the treatment group vs. −17.8 in the control group | |
| Listrat, et al. 14 | 19 treatment 17 control | One year follow-up: pain −16.8 in the treatment group vs. −5.2 in the control group (P = 0.13) Quality-of-life favored = −0.42 in the treatment group vs. +0.18 in the control group (P = 0.047) | |
| Altman, et al.15 | 62 treatment 65 control 63 naproxen | At 26 weeks: slight pain or pain-free, 47.6 percent in the treatment group vs. 33.1 percent in the control group (P = 0.039) vs. 38.9 percent in the naproxen group (P = 0.022) | |
In contrast to these recent trials, which demonstrated no or minimal beneficial effects from intra-articular hyaluronan, other randomized controlled studies12–14 suggest overall beneficial effects of hyaluronan over placebo. Another study15 demonstrated efficacy of hyaluronan in a randomized blinded trial, with the treatment group showing more improvement than the placebo group and a group taking oral naproxen.
In a meta-analysis of eight hyaluronan trials2 involving 971 patients, outcomes in patients treated with hyaluronan were superior to outcomes in patients treated with placebo at the end of the treatment cycles and after six months.
Clinical Studies of Cross-Linked Hylan
Clinical studies have been conducted using cross-linked hylan in the treatment of knee osteoarthritis (Table 2). A summary of four clinical trials performed in Germany using cross-linked hylan16 demonstrated excellent results in 71 percent of hylan-treated patients, compared with 29 percent of placebo-treated patients. After six months, 53 percent of hylan-treated patients still reported excellent pain relief, compared with 22 percent of the placebo-treated patients. In a double-blind, randomized placebo-controlled trial using hylan,17 it was found that 39 to 71 percent of hylan-treated patients were symptom free at 26 weeks compared with 13 to 45 percent of placebo-treated patients. Another study18 compared intra-articular hylan with NSAID therapy in a randomized blinded trial. Hylan was found to be as effective as NSAID therapy at 12 weeks and was superior to NSAID therapy at 26 weeks.
| Study | Number of subjects | Results |
|---|---|---|
| Adams16 | 118 total | At two weeks to six months, treatment group was better (71 percent of the treatment group = excellent vs. 29 percent of the control group). At 6 months, 53 percent of the treatment group = excellent vs. 22 percent of the control group. |
| Wobig, et al.17 | 57 Hylan | At 12 weeks, 47 percent of the treatment group was pain-free vs. 8 percent of the control group (P < 0.001). At 26 weeks, 39 percent of the treatment group was pain-free vs. 13 percent of the control group (P < 0.001). |
| 60 control | ||
| Adams, et al.18 | 32 NSAID | At 12 weeks, all three groups improved from baseline with no significant group differences. At 26 weeks, both Hylan G-F 20 groups were better than the NSAID-alone group. |
| 28 Hylan | ||
| 33 NSAID + Hylan |
In addition, findings from a clinical practice19 showed that 80 percent of 458 knees injected with hylan had a positive response, and the average duration of efficacy was 8.2 months.
Adverse Reactions
In most of the trials of hyaluronan and hylan, rates of adverse reactions have been low (generally zero to 3 percent).7,9,11,12,14–19 No systemic reactions were attributed to hyaluronic acid. Most of the reported adverse reactions consisted of minor localized pain or effusion, which was almost always resolved within one to three days. Case reports of induced pseudogout exist.20 It is unclear whether these local reactions were caused by the hyaluronic acid itself or by the injection procedure. No long-term side effects have been reported.21
Indications
The ideal candidate for intra-articular hyaluronic acid has yet to be defined. Studies are inconclusive regarding the best responders with respect to age, level of osteoarthritis as defined radiographically, level of symptoms and level of physical activity. Intra-articular hyaluronic acid injections should be considered in patients with significantly symptomatic osteoarthritis who have not responded adequately to standard nonpharmacologic and pharmacologic treatments or are intolerant of these therapies (e.g., gastrointestinal problems related to anti-inflammatory medications).2,14,15 Patients who are not candidates for total knee replacement or who have failed previous knee surgery for their arthritis, such as arthroscopic debridement, may also be candidates for viscosupplementation. Total knee replacement in younger patients may be delayed with the use of hyaluronic acid.
A 12-week blinded, randomized trial involving 70 patients compared hylan G-F 20 with a lower-weight hyaluronan.3 The subjects who received hylan G-F 20 had better results on all outcome measures compared with hyaluronan. Based on this trial, hylan G-F 20 may have better clinical efficacy, but further corroborating studies are necessary.
Injection Technique
Hyalgan is supplied in 2-mL vials (one injection per vial) or prefilled syringes, and Synvisc is supplied in 2-mL prefilled syringes. The recommended injection schedule is one injection per week for five weeks for Hyalgan, and one injection per week for three weeks for Synvisc. Repeat courses of viscosupplementation can be performed after six months.
A knee joint can be injected several ways. One approach is to have the patient lie supine on the examination table with the knee flexed 90 degrees (Figure 1). In this position, the anterior portions of the medial and lateral joint lines can easily be palpated as dimples just medial or lateral to the inferior pole of the patella. Often, the medial joint line is easier to palpate and define and can be chosen as the site of injection. Alternatively, the knee joint can be approached with the knee extended, again with the patient lying supine (Figure 2). Most commonly the superolateral edge of the patella is the site of injection, but other quadrants of the knee near the patellar edges can also be chosen. With this approach (knee in extended position), the needle is generally aimed under the patella.
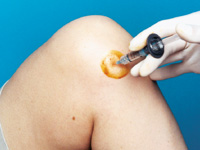
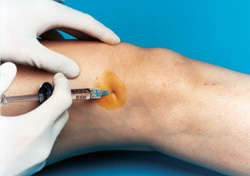
Whichever approach is used, the actual injection site can be marked with a fingernail imprint or the barrel of a pen. Next, sterile preparation with a povidone iodine preparation (Betadine) and alcohol can be performed. A 22- to 25-gauge needle can be used for the injection. Local anesthesia with lidocaine before the injection can be used, but with a small gauge needle this is not always necessary. Alternatively, an ethyl chloride spray can be used for local anesthesia. Following puncture through the skin and into the joint space, the injection is accomplished. If resistance is encountered, redirection of the needle may be necessary.
If effusion is present, aspiration of the joint is recommended before the injection to prevent dilution of the injected hyaluronic acid. The aspiration can be performed at the same site as the injection, as previously described. The same needle can be left in place and used for the aspiration and the injection. In either case, the aspiration may require a larger bore needle, such as an 18- or 20-gauge needle. Following local anesthesia with intradermal lidocaine or ethyl chloride spray, the needle can be placed into the joint for aspiration. When aspiration is completed, hemostat clamps can be used to grasp and stabilize the needle, while the aspiration syringe is detached from the needle. The syringe containing hyaluronic acid can then be attached to the same stabilized needle followed by injection. Alternatively, separate needle sticks can be performed, one for aspiration and another for injection.
No excessive weight-bearing physical activity should take place for one to two days following injection. Otherwise, no specific post-injection instructions are necessary.
Cost
The cost of hyaluronic acid is significant. The average wholesale price for five vials of Hyalgan is $661.00 ($132.20 per vial). For a package of three prefilled syringes of Synvisc, the average wholesale price is $620.00.22 Third-party reimbursement is variable, but Medicare and most insurance companies now cover viscosupplementation.
Final Comment
Viscosupplementation for symptomatic osteoarthritis of the knee is a newly available option. Experience with this treatment is growing as it becomes more widespread among orthopedic surgeons and rheumatologists. Family physicians with the inclination and skills to perform intra-articular injections may also consider this as an option for use in their patients with symptomatic knee osteoarthritis. Although study results are not definitive, data do exist suggesting long-term efficacy in a significant number of patients. Future indications may expand to other joints and other forms of arthritis.