
This is a corrected version of the article that appeared in print.
Am Fam Physician. 2001;63(4):663-673
See patient information handout on infected fingernails and toenails, written by the authors of this article.
Onychomycosis accounts for one third of fungal skin infections. Because only about one half of nail dystrophies are caused by fungus, the diagnosis should be confirmed by potassium hydroxide preparation, culture or histology before treatment is started. Newer, more effective antifungal agents have made treating onychomycosis easier. Terbinafine and itraconazole are the therapeutic agents of choice. Although the U.S. Food and Drug Administration has not labeled fluconazole for the treatment of onychomycosis, early efficacy data are promising. Continuous oral terbinafine therapy is most effective against dermatophytes, which are responsible for the majority of onychomycosis cases. Intermittent pulse dosing with itraconazole is as safe and effective as short-term continuous therapy but more economical and convenient. With careful monitoring, patients treated with the newer antifungal agents have a good chance of achieving relief from onychomycosis and its complications.
Onychomycosis (tinea unguium) is a fungal infection of the nail bed, matrix or plate. Toenails are affected more often than finger-nails.1,2 Onychomycosis accounts for one third of integumentary fungal infections and one half of all nail disease.1 Tinea unguium occurs primarily in adults, most commonly after 60 years of age. The incidence of this infection is probably much higher than the reported 2 to 14 percent.1 Occlusive footwear, locker room exposure and the dissemination of different strains of fungus worldwide have contributed to the increased incidence of onychomycosis.3
Tinea unguium is more than a cosmetic problem, although persons with this infection are often embarrassed about their nail disfigurement. Because it can sometimes limit mobility, onychomycosis may indirectly decrease peripheral circulation, thereby worsening conditions such as venous stasis and diabetic foot ulcers.4 Fungal infections of the nails can also be spread to other areas of the body and, perhaps, to other persons.
Classification of Onychomycosis
DISTAL SUBUNGUAL ONYCHOMYCOSIS
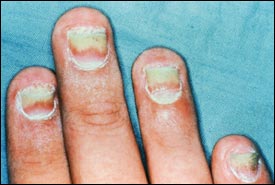
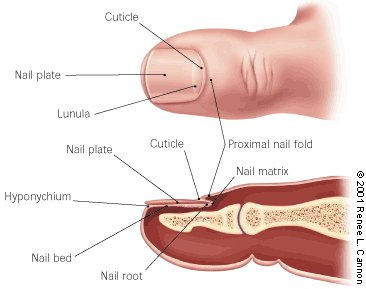
The most common form of tinea unguium is distal subungual onychomycosis, which can also be distal and lateral (Figures 1 and 2). Distal subungual onychomycosis may develop in the toenails, fingernails or both. Some degree of tinea pedis is almost always present. The infection is usually caused byTrichophyton rubrum, which invades the nail bed and the underside of the nail plate, beginning at the hyponychium and then migrating proximally through the underlying nail matrix2,3 (Figure 3). Susceptibility to distal superficial onychomycosis may occur in an autosomal dominant pattern within families.1
WHITE SUPERFICIAL ONYCHOMYCOSIS
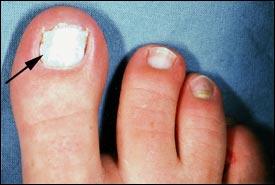
White superficial onychomycosis accounts for only 10 percent of onychomycosis cases.3 The toenails are usually affected (Figure 4). White superficial onychomycosis is caused by certain fungi that directly invade the superficial layers of the nail plate and form well-delineated opaque “white islands” on the plate. As the disease progresses, these patches coalesce to involve the entire nail plate. The nail becomes rough, soft and crumbly. The most common causative agent isTrichophyton mentagrophytes.1–3
PROXIMAL SUBUNGUAL ONYCHOMYCOSIS
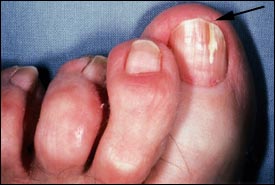
Proximal subungual onychomycosis is the least common form of tinea unguium in healthy persons (Figure 5). It occurs when the infecting organism, usuallyT. rubrum, invades the nail unit through the proximal nail fold, penetrates the newly formed nail plate and then migrates distally. Fingernails and toenails are equally affected.1 This form of onychomycosis usually occurs in immunocompromised persons and is considered a clinical marker of human immunodeficiency virus infection.1 Proximal subungual onychomycosis can also arise secondary to local trauma.1–3
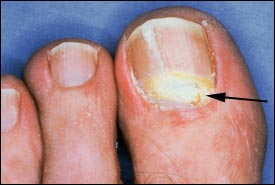
CANDIDAL ONYCHOMYCOSIS
TOTAL DYSTROPHIC ONYCHOMYCOSIS
Total dystrophic onychomycosis may be the end result of any of the four main forms of onychomycosis. This condition is characterized by total destruction of the nail plate.3
Diagnosis
Because fungi are responsible for only about one half of nail dystrophies, the diagnosis of onychomycosis may need to be confirmed by potassium hydroxide (KOH) preparation, culture or histology. Psoriasis, lichen planus, contact dermatitis, trauma, nail bed tumor and yellow nail syndrome may be mistakenly diagnosed as onychomycosis.1,2 A fungal etiology is unlikely if all fingernail or toenails are dystrophic.3
OBTAINING A SPECIMEN
In distal subungual onychomycosis, the concentration of fungus is greatest in the nail bed. Therefore, the nail should be clipped short, and a small curette or number-15 scalpel blade should be used to obtain a specimen from the nail bed as close to the cuticle as possible. A specimen should also be taken from the underside of the nail plate.
In white superficial onychomycosis, a number-15 blade or curette can be used to scrape the nail surface or the white area, and remove infected debris.
In proximal superficial onychomycosis, the healthy nail plate should be gently pared away with a number-15 scalpel blade. A sharp curette can be used to remove material from the infected proximal nail bed as close to the lunula as possible.
In candidal onychomycosis, infected material should be collected from the proximal and lateral nail edges.
Treatment
Historically, the treatment of onychomycosis has been challenging. Orally administered griseofulvin (Grisactin, Gris-Peg) has been available for many years, but its use is limited by a narrow spectrum, the necessity for long courses of treatment and high relapse rates. The oral form of ketoconazole (Nizoral) is much more effective but carries a risk of hepatotoxicity.6
Onychomycosis has long been treated with topical antifungal preparations. However, these agents are inconvenient to use, and results are often disappointing. Treatment using nail avulsion in combination with topical therapy has been somewhat more successful, but this approach can be time-consuming, temporarily disabling and painful.
The U.S. Food and Drug Administration (FDA) has labeled ciclopirox (Penlac) nail lacquer for the treatment of mild to moderate onychomycosis caused byT. rubrum without involvement of the lunula. Although safe and relatively inexpensive, ciclopirox therapy is seldom effective.7
In recent years, treatment outcomes in patients with onychomycosis have improved substantially, primarily because of the introduction of more effective oral antifungal medications.8 Current evidence supports the use of these newer agents as part of individualized treatment plans that consider patient profiles, nail characteristics, infecting organism(s), potential drug toxicities and interactions, and adjuvant treatments.9
Triazole and allylamine antifungal drugs have largely replaced griseofulvin and ketoconazole as first-line medications in the treatment of onychomycosis. These agents offer shorter treatment courses, higher cure rates and fewer relapses.10 Of the newer drugs, terbinafine (Lamisil) and itraconazole (Sporanox) are the most widely used, with fluconazole (Diflucan) rapidly gaining acceptance. These medications share characteristics that enhance their effectiveness: prompt penetration of the nail and nail bed,3,11 persistence in the nail for months after discontinuation of therapy12,13 and generally good safety profiles. Published studies measuring “mycologic cure” (negative KOH preparation or negative cultures) and “clinical cure” (normal nail morphology) have demonstrated the effectiveness of all three medications.
TERBINAFINE
Terbinafine is an allylamine antifungal agent that is active against dermatophytes, which are responsible for the majority of onychomycosis cases. This agent is notably less effective against nondermatophytes, including Candida species and molds.
Adverse effects, including headache, rash and gastrointestinal upset, are reported more often with terbinafine than with placebo. Yet these side effects are uncommon and resolve with discontinuation of the drug.14 Because of its hepatic metabolism, terbinafine has several important drug interactions (Table 1).15–17
| Drug or drug class | Terbinafine (Lamisil) | Itraconazole (Sporanox) | Fluconazole (Diflucan) |
|---|---|---|---|
| Benzodiazepines | Concomitant use of midazolam (Versed) and triazolam (Halcion) contraindicated; use of other benzodiazepines not recommended | Avoid concomitant use because of increased risk of sedation. | |
| Cimetidine (Tagamet) | Increased terbinafine levels possible | Decreased itraconazole absorption | Decreased fluconazole levels possible |
| Gastric pH neutralizers (histamine H2 blockers, proton pump inhibitors, sucralfate [Carafate]) | Monitor for treatment failure because of decreased itraconazole absorption with increased gastric pH. | ||
| HMG-CoA reductase inhibitors | Concomitant use contraindicated because of reported rhabdomyolysis | ||
| Hydrochlorothiazide (Esidrix) and hydrochlorothiazide combinations | Increased fluconazole levels possible | ||
| Oral hypoglycemics (all classes) | Increased hypoglycemia possible | Risk of significant hypoglycemia | |
| Quinidines | Concomitant use contraindicated because of reported ventricular arrhythmias | ||
| Pimozide (Orap) | Concomitant use contraindicated because of reported ventricular arrhythmias | ||
| Rifampin (Rifadin) | Decreased terbinafine levels possible | Decreased fluconazole levels possible | |
| Theophylline | Increased theophylline levels possible | ||
| Warfarin (Coumadin) | Bleeding events reported | Increased risk of bleeding | Increased risk of bleeding |
Rare but serious complications, such as cholestatic hepatitis, blood dyscrasias and Stevens-Johnson syndrome, have been reported in patients treated with terbinafine. Consequently, liver enzyme levels and a complete blood count (including a platelet count) should be obtained before terbinafine is initiated and repeated every four to six weeks during treatment.18 Terbinafine should be discontinued if the aspartate aminotransferase or alanine aminotransferase level becomes elevated to two or more times normal.
The FDA-labeled dosage of terbinafine is 250 mg per day given continuously for 12 weeks to treat toenail infections and for six weeks to treat fingernail infections. Studies have shown that the regimen for toenails results in a mycologic cure rate of 71 to 82 percent and a clinical cure rate of 60 to 70 percent.19,20 Shorter courses and pulse dosing of terbinafine have shown promise in small studies, but data are not yet sufficient to support the use of these regimens.21
ITRACONAZOLE
Itraconazole is a newer triazole medication with a broad antifungal spectrum that includes dermatophytes, many nondermatophytic molds and Candida species. Headache, rash and gastrointestinal upset occur in about 7 percent of treated patients, but hepatic toxicity is rare.22
Because itraconazole is metabolized by the hepatic cytochrome P450 system, significant drug interactions can occur (Table 1).15–17 Notably, concurrent use with quinidines and pimozide (Orap) is contraindicated because of the risk of ventricular arrhythmias. Itraconazole is also contraindicated for concomitant use with 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, such as atorvastatin (Lipitor), because of the increased risk of rhabdomyolysis. In addition, itraconazole should not be taken with some benzodiazepines, such as midazolam (Versed) and triazolam (Halcion), because of exaggerated sedation and potential airway compromise.15
Increased gastric pH decreases the absorption of itraconazole. Therefore, the effectiveness of this antifungal agent can be decreased by histamine H2 blockers such as ranitidine (Zantac) and famotidine (Pepcid), and by proton pump inhibitors such as omeprazole (Prilosec) and lansoprazole (Prevacid). For this reason, itraconazole should be taken with food.
The FDA-labeled dosage of itraconazole is 200 mg once daily taken continuously for 12 weeks to treat toenail infections and for six weeks to treat fingernail infections. The FDA has labeled pulse therapy only for the treatment of fingernail infections. Pulse treatment consists of 200 mg taken twice daily for one week per month, with the treatment repeated for two to three months (i.e., two to three “pulses”).7,8,22,23 This dosage, given in three to four pulses, has also been shown to be effective in the treatment of toenail infections.7,8,22,23 Published studies have demonstrated similar success rates for continuous and pulse therapies, with mycologic cure rates ranging from 45 to 70 percent and clinical cure rates ranging from 35 to 80 percent.22,24,25
Liver enzyme monitoring is recommended before continuous therapy is initiated and every four to six weeks during treatment. No monitoring recommendation is given for pulse therapy.26
FLUCONAZOLE
Like itraconazole, fluconazole is active against common dermatophytes, Candida species and some nondermatophytic molds. Adverse effects, including nausea, headache, pruritus and liver enzyme abnormalities, are reported in approximately 5 percent of treated patients.26 These side effects remit after the discontinuation of fluconazole. The absorption of this drug is not pH sensitive and is not affected by acid suppression or food intake. However, fluconazole has important drug interactions15 (Table 1).15–17
Fluconazole is not currently labeled by the FDA for the treatment of onychomycosis, but early efficacy data are promising.13,27,28 Attention has focused on once-weekly dosing (450 mg), taking advantage of the drug's pharmacokinetics to reduce treatment costs, decrease rates of adverse effects and potentially improve compliance.
In one placebo-controlled study involving patients with fingernail onychomycosis,29 fluconazole in a dosage of 450 mg taken once weekly for three months was associated with a 90 percent clinical cure rate and nearly total mycologic eradication. Lower dosages were slightly less effective. No differences in complication rates were observed between the treatment and placebo groups. Published outcomes data27,28 on the use of fluconazole in toenail fungal infections demonstrated “clinical improvement” (i.e., less than 25 percent of the nail still affected) rates of 72 to 89 percent, compared with 3 percent for placebo.27 Treatment duration in these studies varied from four to nine months, with a small but measurable advantage shown for longer courses.27–29
Treatment guidelines for the newer antifungal medications are provided in Table 2.
| Antifungal agent | Indication | Dosage | Monitoring |
|---|---|---|---|
| Terbinafine (Lamisil) | First-line therapy for dermatophytic infections (most cases of onychomycosis) | 250 mg per day for 6 weeks to treat fingernails and for 12 weeks to treat toenails* | Complete blood count and ALT and AST levels at baseline, then every 4 to 6 weeks during therapy |
| Itraconazole (Sporanox) | Alternative first-line therapy for dermatophytic infections | Continuous therapy: 200 mg per day for 6 weeks to treat fingernails and for 12 weeks to treat toenails* | ALT and AST levels at baseline, then every 4 to 6 weeks during therapy |
| Preferred therapy for nondermatophytic and candidal infections | Pulse therapy: 200 mg twice daily for 7 days per month, with the treatment repeated for 2 to 3 months (“pulses”) to treat fingernails* and for 3 to 4 months to treat toenails† | None recommended | |
| Fluconazole (Diflucan) | First-line therapy for candidal infections but also active against dermatophytes | 150 mg once weekly until nail is normal or acceptably improved (treatment often requires 6 to 9 months)† | None recommended |
| Consider for use in patients with complicated medication regimens |
Comparative Clinical Trials
Much of the published data on the treatment of onychomycosis are of limited clinical use. Many studies have been small and observational, and they have lacked randomization and control subjects. Recently, however, the results of a handful of larger randomized, controlled trials have been published. These studies provide more convincing guidance in choosing appropriate therapy.
In a 1998 study30 of 378 patients with dermatophytic onychomycosis, continuous terbinafine therapy was shown to be more effective than continuous itraconazole therapy in patients with toenail onychomycosis. Intention-to-treat analysis showed nearly 85 percent negative cultures in the treatment group compared with 55 percent in the placebo group, and 65 percent clinical improvement in the terbinafine group compared with 37 percent in the itraconazole group.
Other studies comparing terbinafine and itraconazole had similar findings.31,32 A recent prospective, double-blind, randomized, controlled trial33 compared the use of continuous terbinafine therapy and pulsed itraconazole therapy in 496 patients with toenail fungal infection. This well-designed study showed that terbinafine provided superior clinical and mycologic outcomes up to 15 months after treatment. To date, fluconazole has not been included in published direct-comparison trials.
Most patients in the published studies were infected with dermatophytes, against which terbinafine is most effective. Outcomes data for the treatment of nondermatophytic and candidal onychomycosis are limited, but broader spectrum triazole medications may be more effective against these pathogens.
Cost
Onychomycosis is expensive to treat. Costs include medications, procedures, laboratory tests and health care providers' time, as well as expenses associated with the management of adverse drug effects and treatment failures.
One pharmacoeconomic study34 compared the cost-effectiveness of continuously dosed terbinafine and itraconazole in the treatment of toenail onychomycosis.34 The investigators concluded that continuous terbinafine therapy is less expensive, at a little over one half the price of continuous itraconazole treatment. It should be noted, however, that itraconazole pulse therapy is less expensive than continuous treatment (lower overall drug cost and no need for blood monitoring). Furthermore, the pharmacoeconomic study used national reference pricing and wholesale drug costs. Local laboratory standards, retail pharmacy costs and increasingly common payor formulary considerations may significantly alter individual costs.
Adjuvant Treatments
In addition to oral medications, some patients benefit from other treatments. Surgical or chemical nail avulsion may be useful in patients with severe onycholysis, extensive nail thickening or longitudinal streaks or“spikes” in the nail. These nail changes can be caused by a granulated nidus of infection (dermatophytoma), which responds poorly to standard courses of medical therapy.35,36
Longer courses of antifungal therapy may be useful in patients whose nails grow slowly, who have diminished blood supply to the nail bed as a result of conditions such as peripheral vascular occlusion or diabetes mellitus, or who have total or nearly total nail plate involvement.9
Topical antifungal creams or powders may also be beneficial, especially in patients with concomitant tinea pedis.
To improve treatment outcomes and prevent recurrence, patients should be counseled about proper foot hygiene (Table 3). Patients should be encouraged to wear breathable footwear and 100 percent cotton soc ks when possible. They should be advised to keep their feet dry throughout the day. Similar infection patterns observed in households and patrons of communal bathing facilities suggest a role for foot protection in high-risk areas.21
| Wearing 100 percent cotton socks and changing them often |
| Choosing breathable footwear |
| Protecting feet in shared bathing areas |
| Keeping feet dry throughout the day |
| Recognizing and treating tinea pedis |
| Maintaining and improving chronic health conditions (e.g., controlling diabetes, quitting smoking, etc.) |
There may be a familial predisposition to someT. rubrum infections. In such instances, prophylactic treatment of family members can be considered.37
Treatment Failure and Relapse
Rates of treatment failure can be extracted from published trials, but data on relapse are less readily available. Post-treatment follow-up is long, drop-out rates in many studies are significant or unreported, and most studies have not allowed crossover of treatment regimens. Furthermore, especially in outcomes of clinical improvement (as opposed to cure or fully normal nail appearance), evaluation criteria have not been standardized and often include subjective assessments that are difficult to quantify. Published studies have not specifically addressed the management of treatment failures or relapse.
Despite these difficulties, several measures may be helpful in managing unsuccessful treatment or relapse. The first step is to confirm mycology. If the initial diagnosis was based on a KOH preparation alone, culture of properly collected specimens is mandatory. Culture reports often identify multiple organisms, including possibly nonpathogenic molds, and treatment should be directed at the organism(s) most likely to be causative. A microbiology or infectious disease consultation may be valuable in interpreting the culture report.
Of note, there has been some concern about evolving drug resistance among fungal pathogens, particularly with the widespread use of systemic fluconazole therapy to treat oropharyngeal and recurrent vaginal candidiasis.5 However, the impact of antifungal resistance on the treatment of onychomycosis is not yet clear.
Careful clinical review may identify patient or nail characteristics that are impeding treatment. These factors can be addressed with appropriate medication changes or adjuvant measures. Because of superior efficacy, continuous antifungal therapy may be considered in patients who fail or relapse after pulse therapy.
Onychomycosis in Children
Onychomycosis in children is rare, with an estimated prevalence of 0.2 percent.38 Most often, onychomycosis develops in children with immunosuppression (e.g., acquired immunodeficiency syndrome, chemotherapy, congenital immunodeficiency syndromes), a strong familial history of onychomycosis or extensive cutaneous mycosis (tinea capitis or pedis).
Although griseofulvin remains the mainstay of onychomycosis treatment in children, the efficacy of this drug is variable, and relapse is common. Newly available medications may improve the traditionally mediocre treatment outcomes in this age group.
The FDA has not yet labeled terbinafine for use in children. However, some studies have shown terbinafine to be safe and quite effective in the treatment of tinea capitis, and it is licensed for this purpose in several countries.39 In more limited trials, itraconazole has also been shown to be safe and efficacious in the treatment of tinea capitis.21 If the safety and effectiveness of terbinafine and itraconazole are established over the longer courses needed to treat nail infections, they may become potent first-line therapies for onychomycosis in children.