
Am Fam Physician. 2001;64(1):77-89
A more recent article on benign anorectal conditions is available.
Patients with a wide variety of anorectal lesions present to family physicians. Most can be successfully managed in the office setting. A high index of suspicion for cancer should be maintained and all patients should be questioned about relevant family history or other indications for cancer screening. Patients with condylomata acuminata must be examined for human papillomavirus infection elsewhere after treatment of the presenting lesions. Their sexual partners should also be counseled and screened. Both surgical and nonsurgical treatments are available for the pain of anal fissure. Infection in the anorectal area may present as different types of abscesses, cryptitis, fistulae or perineal sepsis. Fistulae may result from localized infection or indicate inflammatory bowel disease. Protrusion of tissue through the anus may be due to hemorrhoids, mucosal prolapse, polyps or other lesions.
Patients frequently present to family physicians for evaluation of lesions in the anorectal area. Pathologic findings are often discovered during a routine examination or during assessment of symptoms. A thorough physical examination should be performed to detect and evaluate all anorectal lesions. This examination must include abdominal examination, visual inspection of the anal and perineal areas, digital rectal palpation and anoscopic visualization, preferably using an Ive's slotted anoscope (see part I: Symptoms and Complaints). Further testing and examination, including sigmoidoscopy or colonoscopy are indicated in select patients. It is a grave error to automatically assume that every patient who presents with common, mild or occasional symptoms has only a benign condition such as hemorrhoids. Cancer can coexist with benign lesions, so complete assessment is necessary. Colorectal cancer can be cured only if found early. Once cancer is ruled out, more than 90 percent of anorectal complaints can be managed in the primary care physician's office using simple techniques.
Condyloma Acuminatum
Patients are often unaware that condylomata can arise around the anal area (Figure 1). In a sexually active population, the prevalence of DNA of the human papillomavirus (HPV, or “wart virus”) is around 50 percent.1 Once infected with HPV, the entire anogenital tract is involved. Condylomata represent a focal manifestation of a diffuse infection and occur in only a minority of those infected with HPV. Although those who engage in anal intercourse have a higher frequency of perianal condylomata, the majority of patients with perianal condylomata have not engaged in anal intercourse. Infection is believed to occur due to pooling of secretions in the anal area. Condylomata can reach substantial size, and multiple lesions are common. If one lesion is present, a complete genital and anorectal examination is indicated to detect additional growths.
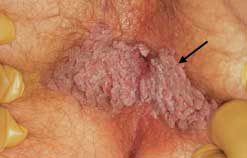
The entire affected area should be soaked for three to five minutes with 3 to 5 percent acetic acid (vinegar). The abnormal warty tissue turns white and can be better distinguished from normal tissue. Magnification devices, such as a colposcope, allow the clinician to observe small lesions that may not otherwise be readily identified. Magnification helps assure that an entire lesion is removed or treated.2
A variety of agents or modalities can be used to successfully treat condyloma acuminatum.3 Trichloroacetic acid (TCA) 85 percent can be applied directly to the lesion. It is not necessary to protect the uninfected area with petroleum jelly because it is difficult to apply and often inadvertently protects the warty tissue. The acid is applied with either the wooden or cotton-tipped end of a cotton swab, depending on the size of the lesion. It burns for about five minutes and must be reapplied after 10 to 14 days.
Treatment with TCA is inexpensive and has an 80 percent efficacy with experienced application. The acid costs less than 50 cents per application. Condylomata may also be subjected to cryotherapy with liquid nitrogen and/or nitrous oxide. The treated area may swell significantly after this treatment. Skin may be sloughed off following treatment, but scarring is uncommon. Cryotherapy is efficacious (63 to 88 percent cure), but several treatments may be needed, especially with large lesions.
Laser therapy can provide cure rates of 60 to 90 percent, but a laser is not readily available to most physicians. Radiofrequency (using the same units that are used for the large loop electrical excision procedure [LEEP]) under colposcopic magnification resolves approximately 80 to 94 percent of condyloma with one treatment. A small wire loop can be used to excise the lesion, or a ball electrode can be used to coagulate the wart. Surgery and electrodesiccation achieve the highest cure rates of all treatments.4
Interferon and fluorouracil (Efudex) are other treatment options. Imiquimod (Aldara), a new immune modifier, is applied three times a week for up to 12 weeks. It is effective in perhaps 50 percent of cases of condyloma acuminatum, with a recurrence rate of 20 percent. The cost per month is around $120.5 Podofilox (Condylox) 0.5 percent comes in a gel and a liquid form. It is applied twice a day for three days, followed by four days of no treatment. This pattern is repeated for six to 12 weeks. The estimated efficacy is around 60 percent,6 and one bottle costs approximately $120.
Whichever treatment modality is used, follow-up anoscopic examination is generally not performed until the external lesions have completely resolved. There is always concern that the virus may be introduced into new and proximal areas by instrumentation. A follow-up anoscopic examination must, however, be performed because occult intra-anal warts are a common cause of recurrence after treatment.
The long-term consequences of HPV infection are of major concern. Infection with HPV has been associated with an increased risk of cervical and anal cancers.7 Receptive homosexuals have 50 times the rate of anal cancers.8 Some experts have suggested that these high-risk patients should have regular Papanicolaou smear screening of the anal canal.9 Female sexual partners of persons with anogenital warts should have annual Pap smears for the rest of their lives, and some experts recommend regular follow-up with colposcopy.
It may be wise to biopsy all “warts” before ablative treatment. Verrucous carcinoma can appear to be a wart. The anal lesion of syphilis (condyloma latum) is usually flat but, if raised, may resemble condyloma acuminatum. Serologic testing for syphilis helps distinguish lesions. Because HPV infection itself indicates exposure to sexually transmitted disease, testing for syphilis and other sexually transmitted diseases maybe indicated.
Fissure
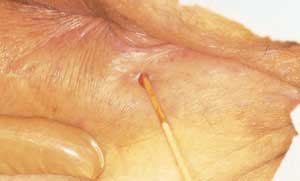
A fissure is a small cut or split in the anoderm (Figure 2). It may be induced by a hard bowel movement or straining at stool. Fissures are most commonly located anterior or posterior to the anus. When fissures are found laterally, syphilis, tuberculosis, occult abscesses, leukemic infiltrates, carcinoma, herpes, acquired immunodeficiency syndrome (AIDS) or inflammatory bowel disease should be considered as causes.
The physical examination is classic in the presence of a fissure. Sphincter tone is markedly increased, and digital examination produces extreme pain. Most fissures can be observed with gentle lateral retraction around the anus. If the patient can tolerate anoscopic examination, a tear may be seen in the mucosa, and frequently there is bleeding.
Treatment for a fissure is quite simple when it is identified within three months of onset (Figure 2). Most patients respond well to rectal suppositories containing a topical corticosteroid and a local anesthetic. As the rectal suppositories melt, the medication soothes the inflamed area, providing symptomatic relief and promoting healing. Some authorities believe that creams are more appropriate than suppositories because suppositories cause pain on insertion and lodge in the pain-insensitive area above the fissure and the dentate line. Whichever modality is selected, adequate relief of pain is essential, and topical xylocaine ointment (Lidocaine) 5 percent may be a useful adjunct treatment. It is also extremely important to keep the stool soft with a high-bulk diet to avoid aggravating the fissure.
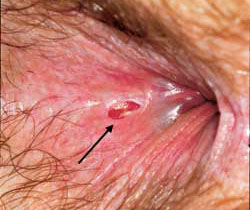
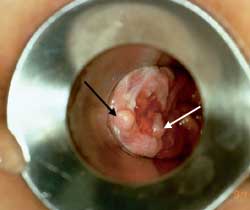
Once a fissure has become chronic (Figure 3), it is more difficult to obtain complete resolution. A sentinel tag or “pile” is frequently observed protruding from the anus. The proximal end of the fissure may contain granulation tissue that is often confused with an anal polyp. The area around the fissure becomes sclerotic and appears white. The sphincter musculature can frequently be visualized at the base of the fissure. Chronic fissures usually require surgical treatment with lateral sphincterectomy.10
The internal sphincter is totally involuntary. In a person with an anal fissure, the internal anal sphincter goes into spasm, and this hypertonicity of the muscle results in pain. Persistent elevation in sphincter tone requires more forceful evacuation of stool, resulting in repeated trauma to the fissure. This vicious cycle forces the fissure open and prevents healing, which in turn exacerbates the sphincter hypertonicity.
The external sphincter is under voluntary control; however, it differs from all other voluntary skeletal muscles in that it maintains a constant tonic contraction at rest. Lateral sphincterotomy procedures incise only the lowest fibers of the internal sphincter. This allows the anal musculature to relax, and the fissure invariably heals. The intact external sphincter maintains continence. Earlier procedures, such as digital stretching, could result in fecal incontinence because of excessive muscular disruption. Appropriately performed surgical lateral internal sphincterotomy has a very low incidence of incontinence.
A nonsurgical treatment for anal fissure is nitroglycerin ointment.11–13 Pharmacists can formulate various preparations with additives such as a local anesthetic, misoprostol or phenytoin to aid the healing process. The most significant adverse effect of nitroglycerin is headache, so the amount used must be limited (usually a “pea-sized drop”). The ointment must be rubbed into the area, not just applied superficially. In one study,14 the application of 0.2 percent nitroglycerin ointment twice daily for six weeks resulted in a 77 percent healing rate, but the efficacy of this treatment has been questioned.15,16 More recently, topical nifedipine17 and diltiazem18 have been used successfully.
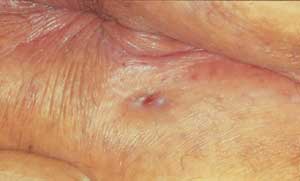
An experimental treatment is botulinum toxin (Botox).19 This treatment causes paralysis of the internal sphincter and allows the fissure to heal. This therapy is expensive, and the results of clinical trials are pending.
Fistula
The most common cause of anal fistula (Figure 4) is cryptoglandular infection. Infections that begin in the anal glands can evolve and present as either abscesses or fistulas. Fistulas are common in patients with Crohn's disease. The track of anal fistulas can be extensive (Figure 5). Flexible sigmoidoscopic examination is indicated to evaluate the mucosa of the distal colon for signs of inflammatory bowel disease. The index of suspicion for Crohn's disease is increased by a history of episodes of diarrhea, abdominal cramping and weight loss, and the appearance, location and multiplicity of the fistulas.
Patients with fistulas are generally referred to a specialist for treatment. In addition to simple fistulotomy treatments include cutting or draining setons, endo-anal mucosal advancement flaps, sliding cutaneous advancement flaps, fistulectomy with muscle repair and fibrin glue injection.
Abscesses
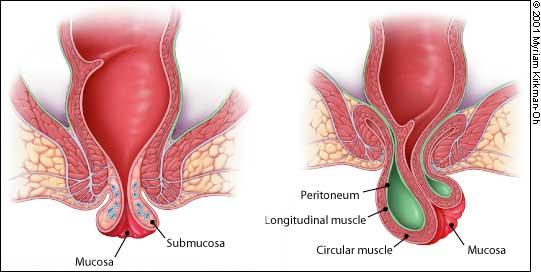
Abscesses also begin as an infection in the anal glands. The suppurative process then tracks through the various planes in the anorectal region (Figure 6). The infection can present at the anal verge as a perianal abscess. These abscesses are easily drained in the office under local anesthesia.
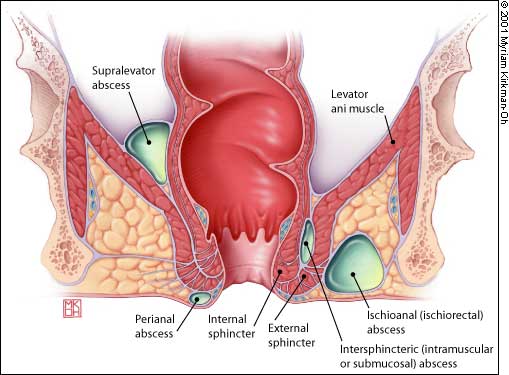
The infection may track through the internal and external sphincter muscles to enter the ischiorectal space. These large ischiorectal abscesses are visible on the surface of the buttocks and are best managed by surgical drainage accomplished in the operating room. Infection that tracks cephalad between the inner circular and outer longitudinal muscle layers of the anorectal wall presents as an intramuscular or “submucosal” abscess. The patient complains of anal pain, and the most significant finding is a highly painful bulge that is palpated within the rectum. These abscesses are difficult to diagnose and require a high index of suspicion. Treatment options include surgical drainage into the rectum.
Finally, a supralevator abscess can arise from cryptoglandular anal disease or from an abdominal suppurative condition. In supralevator abscess, it is particularly important to identify and treat the process leading to abscess formation as well as to relieve the abscess itself by surgical drainage.
Cryptitis
Cryptitis is a localized infection of one of the anal glands. This unusual condition is identified anoscopically as a pearl of pus beading up from the crypt at the level of the dentate line. Treatment is obliteration of the gland, which necessarily involves an internal sphincterotomy. Fistulae and abscesses can develop with untreated prolonged infection.
Perineal Sepsis
Pain in the perineal area, fever and inability to void form the classic triad of signs of perineal sepsis. The most common cause is advanced cryptoglandular infection resulting in a necrotizing perineal infection. This is a medical emergency requiring immediate hospitalization and treatment, including surgical debridement and high dosages of broad-spectrum antibiotics. Rarely, perineal sepsis occurs as a complication of rubber band ligation of internal hemorrhoids.
Proctitis/Ulcerations/Inflammatory Bowel Disease
The anorectal area can be involved in several infectious and inflammatory processes. Symptoms vary depending on the specific infection or pathologic process but typically include rectal discomfort, tenesmus, rectal discharge and constipation. The rectal mucosa is often friable, and a mucopurulent discharge may be present. Herpes infection of the anal area presents as small, painful vesicles followed by ulceration and resolution over seven to 10 days. At times, aphthous-type lesions can be observed on anoscopy. Biopsy is warranted for diagnosis. Neisseria gonorrhoeae, Chlamydia trachomatis and syphilis can also infect the anus. Crohn's disease and, more commonly, ulcerative colitis can involve the rectal area, presenting as proctitis or fistulae.
Hemorrhoids
A comprehensive review of the evaluation and treatment of hemorrhoids (Table 1) has previously been published.20 Hemorrhoids are classified as internal, external and mixed, based on their site of origin. External hemorrhoids begin below the dentate line, while internal hemorrhoids originate above the dentate line (Figure 7). Mixed hemorrhoids can indicate lesions that originate at the dentate line, or the term can be used to describe the presence of both internal and external hemorrhoids.
| External |
| Observation; local measures |
| Surgical excision |
| If thrombosed: observation, incision or excision |
| Internal |
| Band ligation |
| Infrared coagulation |
| Radiofrequency treatment |
| Sclerotherapy |
| Surgical excision/laser |
| Anal tags |
| Surgical excision |
External hemorrhoids can cause a bulge at the time of defecation. More commonly, patients present when they thrombose (Figure 8). Thrombosed hemorrhoids are treated by incision under local anesthetic to remove the clot. Conservative therapy with sitz baths and pain medication may be indicated, although recovery is prolonged. Simple incision and expression of the clot has a higher immediate recurrence rate, and many practitioners recommend excising an ellipse over the surface of the hemorrhoid or completely excising the clotted hemorrhoid at the time of occurrence. Suture closure is generally indicated. Some clots are so large that office excision is not possible.
Whichever surgical technique is used, the entire thrombus must be removed. Hemorrhoids contain septate channels and, if the entire clot is not removed, thrombosis will reaccumulate around the nucleus of retained clot. Most thrombosed external hemorrhoids are of a suitable size for office excision.
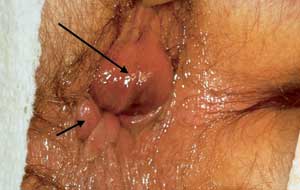
Internal hemorrhoids are graded from I to IV based on the degree of prolapse (Figure 9). Grade I internal hemorrhoids bulge with defecation. In grade II hemorrhoids, prolapse occurs with defecation, but the lesions recede spontaneously. Grade III hemorrhoids require digital replacement after prolapsing. Hemorrhoids in grade IV cannot be replaced once they are prolapsed. The treatment of internal hemorrhoids has been markedly simplified in the past 15 years (Table 1). Two meta-analyses have concluded that preferred treatments for internal hemorrhoids include rubber band lig-ation21 and infrared coagulation (IRC; Redfield Corp.).22 Almost all hemorrhoid symptoms (approximately 95 percent) that present to primary care physicians should be treatable in the office.23–27 Equipment for rubber band ligation is the least expensive to acquire, while infrared coagulation is the simplest therapy to apply.
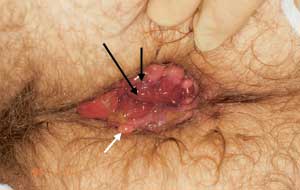
Band ligation can be used to treat most grade II and III, and some grade IV internal hemorrhoids. Infrared coagulation is suitable for grades I and II, and some grade III lesions. The radiofrequency treatment unit (Bicap; Circon Corp.) can be used for grades I through IV. Sclerotherapy is now rarely used in the United States for the treatment of internal hemorrhoids, with the possible exception of patients with bleeding disorders or those taking anticoagulation medication.
Prolapsed, irreducible hemorrhoids can become gangrenous and require emergency surgical intervention.
Mucosal Prolapse and Full-Thickness Rectal Prolapse (Procidentia)
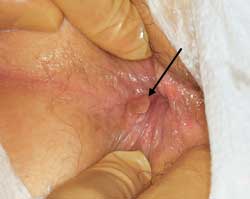
Patients may present with protrusion of tissue through the anus but, on examination, little evidence of hemorrhoidal disease can be found. In these circumstances, the mucosa of the rectum or anal canal or the entire thickness of the rectal wall may have prolapsed into the anal outlet. Mucosal prolapse is complete eversion of the anal mucosa. On the other hand, rectal prolapse is a full-thickness evagination of the rectal wall outside the anal opening. In rectal prolapse, concentric folds are found on the surface of the prolapsed tissue, whereas radial folds are noted on the surface in mucosal prolapse. With full-thickness rectal prolapse, the anus is in its normal anatomic position, whereas in mucosal prolapse the anoderm is also everted and visible externally (Figure 10). Accordingly, in full-thickness rectal prolapse, there is a sulcus between the anus and the everted bowl. This is absent in mucosal prolapse.
In either situation, the treatment is usually surgical. Full-thickness rectal prolapse usually requires a resection of the bowel. For mucosal prolapse, the excision of the redundant mucosa is all that is required.
Anal Tags
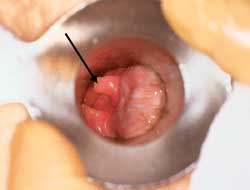
Anal tags (Figure 11) are generally asymptomatic and often are the remnants of previously thrombosed external hemorrhoids. When tags are symptomatic, as a result of itching, anxiety or hygienic problems, they can be removed. If tags are small, local anesthetic is injected, the area is excised and the site is either sutured or left open to heal by secondary intention. If extensive, excision may need to be undertaken in the operating room. Any surgery below the dentate line causes considerable postoperative pain.
Hypertrophied Papillae
At times a movable mass can be palpated in the anal area and, on anoscopic examination, a thickened anal papilla is visualized at the dentate line. This can be confused with a polyp. Hypertrophied papillae are generally white, firmer on digital examination than polyps, and located at the dentate line (Figure 12). Treatment is generally not indicated for confirmed hypertrophied papillae.
Stricture/Stenosis
In most cases, when patients complain of difficulty passing stool or that the “opening seems small,” no pathologic conditions are identified. True stricture or stenosis of the anal outlet can occur secondary to prior anal surgery or radiation therapy, and can be a long-term complication of chronic anal fissure, inflammatory bowel disease, perianal dermatopathy anal cancer or trauma. Surgical therapy for anal stenosis is required when stool softeners, osmotic agents and lubricants are no longer effective in ensuring adequate passage of stool.
The treatment for symptomatic benign anal stenosis is lysis of the fibrous cicatrix, with or without a concomitant sphincterotomy. The anal canal can be enlarged further with the addition of an advancement flap anoplasty. Early postoperative strictures can be gently dilated daily with a lubricated digit or with Hegar dilators. Gentle and judicious dilatation under anesthesia may also be justified in patients with strictures resulting from radiation therapy or inflammatory bowel disease.
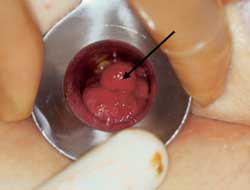
Polyps
Although more commonly found higher in the large bowel on sigmoidoscopy or colonoscopy polyps may also be seen on anoscopy or protruding from the anus (Figure 13). Adenomatous polyps (tubular, tubulovillous and villous) are precursors to cancer and are also termed “neoplastic polyps.”28 They must be distinguished from hyperplastic polyps, which are usually quite small (less than 5 mm) and have no neoplastic potential,29 and the inflammatory pseudopolyps associated with inflammatory bowel disease. The finding of an adenomatous polyp, regardless of size, is an indication for a total colonic examination.30 Biopsy is required to distinguish hyperplastic polyps from small adenomatous polyps.
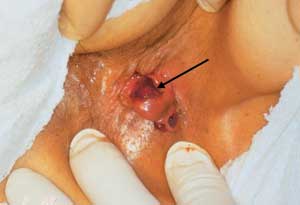
Chronic Solitary Ulcer
Although rare, chronic solitary ulcer (Figure 14) may occur in patients of all ages. The patient presents with a mass that is ulcerated. The appearance closely resembles cancer. The cause of chronic solitary ulcer is unknown. If the lesion is at the apex of a prolapsing hemorrhoid, excision is the treatment of choice, and recurrence is unusual. If, however, the ulcer is located in the midrectum, excision is generally unsatisfactory. Chronic solitary ulcer is usually associated with defecation disorders and often confused with or mistaken for rectal cancer. The lesion must be biopsied to make sure that it is not neoplastic.31
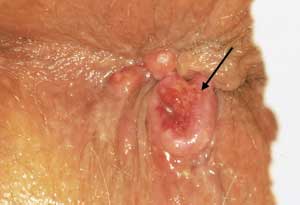
Cancer of the Anus and Rectum
Anal cancer can cause many different symptoms or be an incidental finding on rectal examination. Pain is usually absent, and rectal bleeding is inconsistent. An external or internal mass may be palpable. Some lesions are so soft that they are missed on palpation. Anal cancer can take several forms (Figure 15), such as ulcers, polyps or verrucous growths. Anal cancers are staged and treated differently from rectal cancers and have a distinct biologic behavior. Most anal cancers respond well to treatment with combined chemotherapy and pelvic radiation.
Colorectal cancer is almost always treated surgically. Once these cancers become symptomatic, the prognosis worsens. When it is found at early stage I, 95 percent of patients with colorectal cancer survive at least five years. Once stage IV (distant metastases) is reached, the likelihood of five-year survival is less than 10 percent. It is imperative that any patient who presents with rectal bleeding have an immediate digital and anoscopic examination. If the site of the bleeding is not conclusively identified, further investigations are indicated because of the high incidence of colorectal cancer in patients with rectal bleeding.
Patients with familial polyposis syndromes, who have hundreds of polyps, can present with rectal bleeding in their teenage years. Screening of family members begins at puberty with colonoscopy. Genetic testing is now available to detect polyposis as well as hereditary nonpolyposis colon cancer syndrome.32 In the latter condition, colonoscopy of family members is indicated at 21 years of age. A family history of colon cancer should be sought at all routine physical examinations. Anyone with a first-degree relative with colon cancer or adenomatous polyps diagnosed before age 60 should have the entire bowel screened for colon cancer at age 40, or 10 years before the age of diagnosis in the relative26 (Table 2). Currently, only 20 percent of eligible patients in the United States are adequately screened for colon cancer.33
| Risk category | Recommendation* | Age to begin | Interval | |
|---|---|---|---|---|
| Average risk | ||||
| All patients age 50 and over not in the categories below | FOBT plus flexible sigmoidoscopy | 50 | FOBT every year and flexible sigmoidoscopy every 5 years | |
| or | ||||
| TCE† | 50 | Colonoscopy every 5 to 10 years or DCBE every 5 to 10 years | ||
| Moderate risk | ||||
| Patients with single, small (<1 cm) adenomatous polyps | Colonoscopy | At time of initial polyp diagnosis | Colonoscopy within 3 years after initial polyp removal; if normal, as per average risk recommendations above | |
| Patients with one large (≥1 cm) adenomatous polyp or multiple adenomatous polyps of any size | Colonoscopy | At time of initial polyp diagnosis | Colonoscopy within 3 years after initial polyp removal; if normal, colonoscopy every 5 years | |
| Personal history of curative-intent resection of colorectal cancer | Colonoscopy | Within 1 year after resection | If normal, colonoscopy in 3 years; if still normal, colonoscopy every 5 years | |
| Colorectal cancer or adenomatous polyp in first-degree relative before age 60 | Colonoscopy | 40 (or 10 years before the youngest case in the family, whichever is earlier) | Every 5 years | |
| Colorectal cancer or adenomatous polyps in two or more first degree relatives of any age | Colonoscopy | 40 (or 10 years before the youngest case in the family, whichever is earlier) | Every 5 years | |
| Colorectal cancer in any other relatives (not included above) | As per average risk recommendations above; may consider beginning screening before age 50 | — | — | |
| High risk | ||||
| Family history of familial adenomatous polyposis | Early surveillance with endoscopy, counseling to consider genetic testing and referral to a specialty center | Puberty | If familial polyposis is confirmed, consider colectomy; otherwise, endoscopy every 1 to 2 years | |
| Family history of hereditary nonpolyposis colon cancer | Colonoscopy and counseling to consider genetic testing | 21 | Every 2 years until age 40, then every year | |
| Inflammatory bowel disease‡ | Colonoscopy with biopsies for dysplasia | 8 years after the start of colitis | Every 1 to 2 years | |