
Am Fam Physician. 2002;65(5):915-921
According to the American College of Obstetricians and Gynecologists, it has become standard in prenatal care to offer screening tests for neural tube defects and genetic abnormalities. There have been some changes in the recommended method of prenatal screening over the past few years, and research to improve detection rates with better combinations of maternal serum analytes is ongoing. The issues facing physicians are the sensitivity and specificity of multiple serum analyte combinations. The current maternal serum analytes in use in most areas are alpha-fetoprotein (AFP), human chorionic gonadotropin (hCG) and unconjugated estriol. Measurement of AFP alone can detect the vast majority of neural tube defects and a small portion of trisomy 21–affected pregnancies in patients of all ages. Adding hCG and unconjugated estriol to this screen increases the rate of detection of trisomies 21 and 18. Counseling patients about the risks and benefits of such screening is important to provide a balanced discussion of screening issues.
Prenatal screening is an issue that has become more important over the past few years. Most elements of standard prenatal care are relatively straightforward and easy for patient to understand and accept, but screening and diagnostic testing for chromosomal abnormalities remain confusing, emotionally charged and fraught with uncertain risks. The most commonly used test for genetic diagnosis is amniocentesis, but the rate of spontaneous fetal loss related to amniocentesis averages about one in every 200 procedures.1 Because of this risk, serum analyte testing has become an important, noninvasive first step in detecting patients at risk for congenital abnormalities. Current maternal serum analyte screening helps identify women at risk for neural tube defects (NTDs), trisomy 21 and trisomy 18.
NTDs are some of the most common serious fetal malformations in the United States, second only to cardiac defects.2 The incidence of NTDs is 1.2 for every 1,000 births.1 These defects include anencephaly, spina bifida and encephalocele. Spina bifida has the third highest lifetime cost of any congenital anomaly.3
Trisomy 21 (Down syndrome) is associated with mental retardation, malformation of the heart, gastrointestinal tract, eyes and ears, and early Alzheimer's disease.1 The overall risk of having an affected fetus is one in 1,000 live births. The second trimester risk is one in 270 in women 35 to 40 years of age, and one in 100 in women older than 40 years.1 It has long been accepted that women who are 35 years or older at the time of delivery should be offered prenatal diagnosis with amniocentesis or chorionic villus sampling.4 Although the risk for trisomy 21 increases with maternal age, an estimated 75 percent of affected fetuses are born to mothers younger than 35 years.1 Because of this risk, it is important to provide pregnant women who are younger than 35 years with noninvasive screening for this trisomy.
Maternal Serum Analyte Screening Tests
Over the past decade, many serum analytes have been studied as screening tests for fetal anomalies. Current maternal serum testing uses three distinct analytes to screen for trisomy syndromes in low-risk patients while incorporating the detection of NTDs. The hormones tested are alpha-fetoprotein (AFP), human chorionic gonadotropin (hCG) and unconjugated estriol.
ALPHA-FETOPROTEIN
AFP was first recognized as a fetal-specific globulin in 1956. It is synthesized in the yolk sac, gastrointestinal tract and liver of the fetus. Fetal plasma levels peak at 10 to 13 weeks' gestation and decline progressively until term,7 while maternal levels peak in the third trimester.2 Laboratory measurements of AFP levels are reported as multiples of the median (MOM). Physicians need to understand how their reference laboratories report AFP results.
There are various reasons for and syndromes associated with elevated and depressed AFP levels (Tables 1 and 2).2 The most common reason for an abnormal AFP level is an inaccurate estimated gestational age. Using AFP in conjunction with ultrasonography to confirm dates, 21 percent of trisomy 21 pregnancies and 5 percent of normal pregnancies would be selected for amniocentesis.8 AFP is better at detecting NTDs than ultrasonography, and it is the only marker in the triple screen useful for NTD detection. It can uncover 90 percent of anencephalic pregnancies and 80 percent of spina bifida cases.2 Markedly elevated levels of AFP indicate that the fetal integument is not intact.6 A MOM of 2.0 to 2.5 or greater for gestational age shows an increased risk of NTDs and merits further evaluation by ultrasound examination or repeat AFP measurements.2 If an NTD exists, the level will continue to increase.6
| Gestational age younger than calculated | |
| Spina bifida | |
| Anencephaly | |
| Congenital skin defects | |
| Pilonidal cysts | |
| Abdominal wall defects | |
| Gastrointestinal defects | |
| Obstruction | |
| Liver necrosis | |
| Cloacal exstrophy | |
| Cystic hygroma | |
| Sacrococcygeal teratoma | |
| Renal anomalies | |
| Urinary obstruction | |
| Polycystic kidney | |
| Absent kidney | |
| Congenital nephrosis | |
| Osteogenesis imperfecta | |
| Low birth weight | |
| Oligohydramnios | |
| Multiple gestation | |
| Decreased maternal weight | |
| Gestational age older than expected |
| Chromosomal trisomies |
| Hydatidiform mole |
| Fetal demise |
| Increased maternal weight |
HUMAN CHORIONIC GONADOTROPIN
A complex glycoprotein, hCG is produced exclusively by the syncytiotrophoblast shortly after implantation into the uterine wall. It increases rapidly in the first eight weeks of gestation.9 It then decreases steadily until 20 weeks, when it plateaus.6 Maternal weight and parity affect hCG levels.2 An increased hCG level appears to be the most sensitive marker for detecting trisomy 21.10,11 A low hCG level is associated with trisomy 18.6 The hCG levels are normal in NTDs. By providing amniocentesis to all women older than 35 years and younger than 35 years with an age-adjusted AFP level indicating a risk of trisomy 21 equivalent to that of a 35 year old, 25 to 50 percent of cases of trisomy 21 can be detected.2 The addition of hCG to the AFP screen increases detection of Down syndrome by about 40 to 50 percent over AFP alone.11
UNCONJUGATED ESTRIOL
Unconjugated estriol is produced by the placenta from precursors provided by the fetal adrenal glands and the liver.6 It increases steadily throughout pregnancy to a higher level than is normally produced by the ovaries.5 Unconjugated estriol levels are decreased in trisomy 21 and trisomy 18.2 The addition of unconjugated estriol to hCG and AFP screening increases the detection of trisomy 21 in women younger than 35 years while only slightly increasing the false-positive rate.11,12
TRIPLE SCREEN
The triple screen is most accurate if done between 16 and 18 weeks of gestation, but it can be done from 15 to 22 weeks of gestation.2 The test costs from $85 to $240, depending on the laboratory. Triple screening should not replace amniocentesis or chorionic villous sampling in pregnancies at high risk for trisomy 21 (e.g., advanced maternal age). If amniocentesis is omitted in the care of pregnant women older than 35 years, 20 to 30 percent of fetuses affected by trisomy 21 will be missed with the triple analyte screening method.11 In women at high risk who refuse amniocentesis, the triple screen is suggested as a noninvasive alternative.4,13
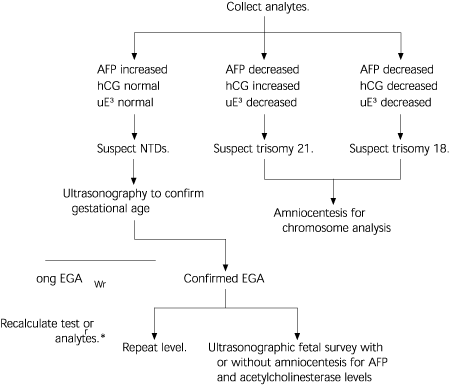
Appropriate diagnostic testing after a positive screen result depends on the analyte levels (Table 3).2 If the AFP level is high, an ultrasound examination (if not already done) is warranted to confirm gestational age or search for defects. This examination may be followed with amniocentesis, depending on the result of the ultrasound examination. Because the AFP level is almost persistently elevated in NTDs, a repeat measurement of the serum AFP level is sometimes obtained before amniocentesis. If the analytes indicate trisomy, amniocentesis with chromosome analysis would be the next screening step (Figure 1).4 All three tests together detect 60 to 70 percent of cases of trisomy 21, with an amniocentesis rate of 5 percent.4,11,14 This detection rate is an increase of two to three times over that of AFP screening alone.11
| Anomaly | AFP | HCG | uE3 |
|---|---|---|---|
| NTDs | Increased | Normal | Normal |
| Trisomy 21 | Decreased | Increased | Decreased |
| Trisomy 18 | Decreased | Decreased | Decreased |
The risk cutoff selected will affect the detection rate and the false-positive rate of the test. Traditionally, a risk cutoff of one in 270, the second trimester risk of a 35-year-old woman for trisomy 21, has been used. If a lower cutoff is selected, the number of false positives is decreased, as well as the detection rate. The best risk cutoff to use is the one that gives the best balance of detection versus false positives. Several studies15–17 that have looked at this issue have found that one in 190 is probably the cutoff that gives the best balance. This cutoff produces a detection rate of about 70 percent for trisomy 21 and 60 percent for all trisomies, with a false-positive rate of 20 percent.
There are multiple advantages of knowing if a fetus is affected prenatally (Table 4).15,18 In addition to allowing the parents to decide whether they wish to continue the pregnancy, such knowledge may prevent unnecessary trauma to the fetus if an operative delivery is indicated. Prenatal treatment is becoming available for some NTDs and the appropriate neonatal team can be assembled at the time of delivery to care for the infant.
| In most cases, the news will be good and may reassure the patient. |
| Some patients may decide to terminate the pregnancy when faced with a lethal abnormality. |
| Anomaly detection may allow specialized antenatal treatment and change perinatal treatment. |
| If the patient chooses not to terminate the pregnancy, she might still find it reasonable to avoid a cesarean delivery for fetal distress in a child with a lethal anomaly. |
| It is much gentler to the parents to learn of anomalies early rather than during the stressful, usually happy time of labor and delivery. |
| The parents have time to prepare emotionally and financially. |
| The family can educate themselves about the anomaly. |
ADDITIONAL SCREENING TESTS
Inhibin A, a glycoprotein synthesized by the gonads, corpus luteum, decidua and placenta, is the latest maternal serum analyte to show promise as a screening agent in clinical studies. An initial retrospective study19 suggested that AFP, hCG and inhibin A analytes might be a better combination than AFP, hCG and unconjugated estriol. A more recent prospective study17 of women with an average age of 35.9 years demonstrated that the addition of inhibin A to the triple screen was actually the best combination of analytes. The combination of all four analytes detected 85 percent of trisomy 21 cases at a risk cutoff of one in 270 and one in 190, while the triple screen detected only 69 percent in the study population, at a risk cutoff of one in 190.19 With the four-analyte combination, decreasing the risk cutoff from one in 270 to one in 190 decreased the false-positive rate from 24 to 19 percent.17 Screening with all four markers is now available at some clinical laboratories and is likely to be more widely available in the near future if these results can be verified in younger women.
Counseling
Beyond the technical aspects of maternal serum analyte screening lies the human aspect. Patients need to understand what screening tests are being offered and how they may affect them. The physician needs to provide patients with the risks and benefits of performing these screens. While appropriate counseling before testing is essential, it is not always done.16 Counseling should be nondirective and include all relevant information (Table 5).17 It is important that patients understand that this is a screening test and that a positive or negative result is not an absolute indication that something is or is not wrong with their infant. It is also important that patients not be required to make definitive decisions about how they will respond to the results before the testing occurs. Some patients change their minds when faced with an abnormal result or as a result of counseling before further testing.20–22
| Conditions detectable by the screen |
| Diagnostic test available if the screen is positive |
| Risk to mother and child of the test being performed |
| Accuracy of the test |
| Limitations of the test |