
Am Fam Physician. 2011;84(1):123-125
A more recent Practice Guideline on CDC guidelines on sexually transmitted diseases is available.
| Guideline source: Centers for Disease Control and Prevention |
| Evidence rating system used? No |
| Literature search described? No |
| Published source:MMWR Recommendations and Reports, December 17, 2010 (correction published in January 14, 2011 issue) |
| Available at: http://cdc.gov/mmwr/preview/mmwrhtml/rr5912a1.htm |
The Centers for Disease Control and Prevention (CDC) recently updated its guidelines on the treatment of sexually transmitted diseases (STDs). The new guidelines focus on treatment, but also discuss prevention strategies and diagnostic recommendations. Prevention and control of STDs are based on five major strategies: (1) education and counseling of high-risk persons on changing their sexual behaviors and using recommended prevention services; (2) identification of asymptomatically infected persons and of symptomatic persons who are unlikely to seek diagnostic and treatment services; (3) effective diagnosis, treatment, and counseling of infected persons; (4) evaluation, treatment, and counseling of sex partners of persons who are infected with STDs; and (5) pre-exposure vaccination of persons at risk of vaccine-preventable STDs (human papillomavirus and hepatitis A and B).
Updated Recommendations
EVALUATION FOR CERVICITIS AND TRICHOMONIASIS
Cervicitis may be a sign of upper genital tract infection; therefore, women who present with a new episode of cervicitis should be assessed for signs of pelvic inflammatory disease and tested for Chlamydia trachomatis and Neisseria gonorrhoeae infections using nucleic acid amplification testing. Women with cervicitis should also be evaluated for bacterial vaginosis and trichomoniasis. Because the sensitivity of microscopy to detect Trichomonas vaginalis is relatively low, symptomatic women with cervicitis and negative microscopy for trichomonads should be tested further. Infection with herpes simplex virus type 2 (HSV-2) has been associated with cervicitis; however, the usefulness of testing for HSV-2 in this setting is not known.
Because of the high prevalence of trichomoniasis, women seeking care for vaginal discharge should be tested. Screening can be considered for women at high risk of infection (i.e., those with new or multiple sex partners or a history of STDs, and those who exchange sex for payment or use injection drugs). Approved tests for vaginal secretions include the OSOM Trichomonas Rapid Test and the Affirm VP III test, which have a sensitivity of greater than 83 percent and a specificity of greater than 97 percent. Amplicor, a polymerase chain reaction assay for detection of C. trachomatis and N. gonorrhoeae infections, has been modified for T. vaginalis detection in vaginal or endocervical swabs and in urine from men and women. Sensitivity ranges from 88 to 97 percent, and specificity is 98 to 99 percent. The Aptima T. vaginalis Analyte Specific Reagents test can detect T. vaginalis RNA by transcription-mediated amplification, with a sensitivity of 74 to 98 percent and specificity of 87 to 98 percent. Culture for T. vaginalis is another option.
Wet preparation is not a sensitive test for trichomoniasis in men, and no approved point-of-care tests are available. Culture testing of urethral swab, urine, or semen is an option; however, nucleic acid amplification testing is more sensitive. Oral and rectal testing for T. vaginalis is not recommended.
BACTERIAL VAGINOSIS
Treatment is recommended for women with symptoms of bacterial vaginosis, and may reduce the risk of acquiring C. trachomatis, N. gonorrhoeae, human immunodeficiency virus, and other viral STDs. Recommended antimicrobial regimens include metronidazole (500 mg orally twice per day for seven days, or 5 g of 0.75% gel administered intravaginally once per day for five days) or clindamycin (5 g of 2% cream administered intravaginally at bedtime for seven days). Alternative regimens include tinidazole (Tindamax; 2 g orally once per day for two days, or 1 g orally once per day for five days) or clindamycin (300 mg orally twice per day for seven days, or 100 mg of ovules administered intravaginally at bedtime for three days). Patients should abstain from intercourse or use condoms consistently during treatment.
GENITAL WARTS
Treatment of genital warts should be guided by patient preference, available resources, and physician experience. None of the available treatments has been proven superior to others, and no single treatment is ideal for all patients. Factors that influence treatment selection include the size, number, and morphology of the warts; anatomic site; adverse effects of treatment; convenience; and cost. Foregoing treatment to wait for spontaneous resolution of warts is acceptable in select patients.
Treatments are classified as patient-applied or physician-administered (Table 1). To ensure that patient-applied therapies are effective, patients must comply with the treatment regimen and must be capable of identifying and reaching all genital warts. Follow-up visits are not required.
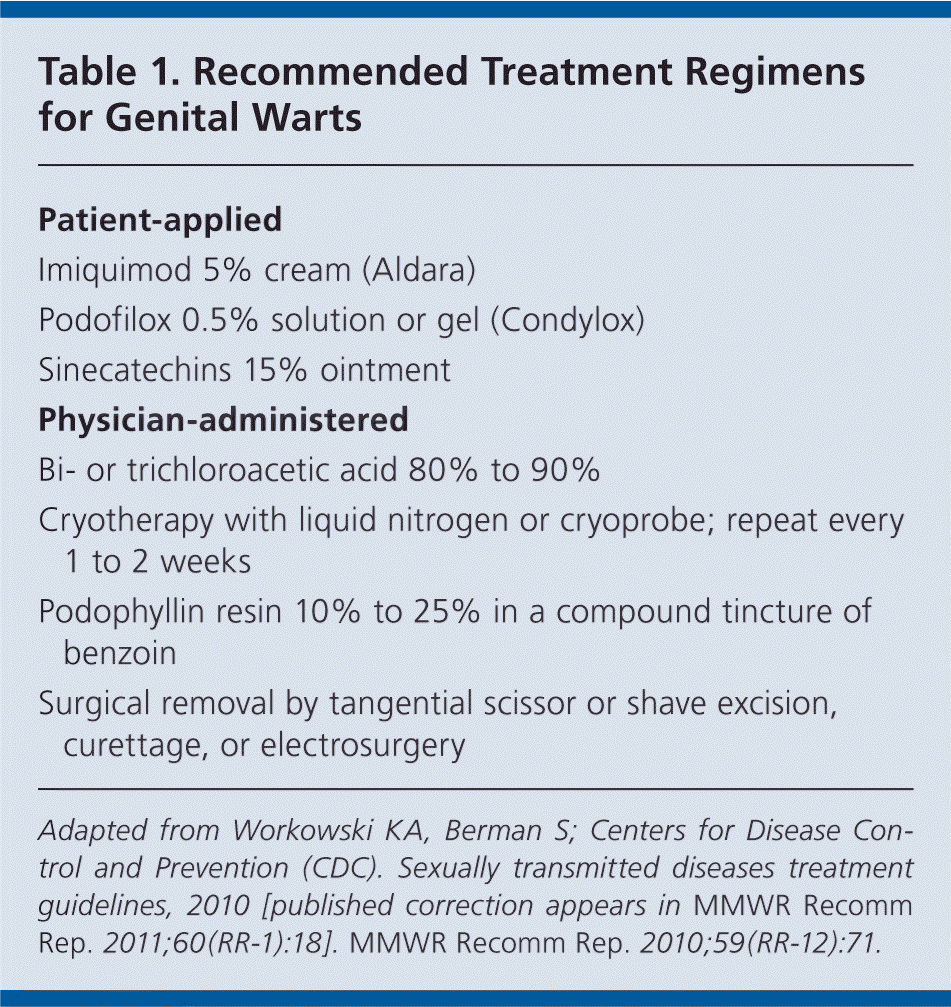
| Patient-applied |
| Imiquimod 5% cream (Aldara) |
| Podofilox 0.5% solution or gel (Condylox) |
| Sinecatechins 15% ointment |
| Physician-administered |
| Bi- or trichloroacetic acid 80% to 90% |
| Cryotherapy with liquid nitrogen or cryoprobe; repeat every 1 to 2 weeks |
| Podophyllin resin 10% to 25% in a compound tincture of benzoin |
| Surgical removal by tangential scissor or shave excision, curettage, or electrosurgery |
CHLAMYDIA DURING PREGNANCY
Many of the recommended and alternative treatments for chlamydia are contraindicated in pregnant women. However, azithromycin (Zithromax) is effective and safe during pregnancy. It should be given as a single 1-g dose. Amoxicillin (500 mg orally three times per day for seven days) is also a recommended therapy during pregnancy. Alternative regimens include erythromycin (500 mg orally four times per day for seven days, or 250 mg orally four times per day for 14 days) or erythromycin ethylsuccinate (800 mg orally four times per day for seven days, or 400 mg orally four times per day for 14 days). Repeat testing, preferably by nucleic acid amplification assay, is recommended three weeks after therapy is completed to ensure chlamydial eradication. Women with chlamydia diagnosed during the first trimester of pregnancy should also be retested three months after treatment. Women who are younger than 25 years or at increased risk of chlamydia (i.e., those who have more than one sex partner or a new partner) should be retested during the third trimester.
GONORRHEA
Treatment of gonorrhea is complicated by the ability of N. gonorrhoeae to develop resistance to antimicrobial therapies. Quinolone-resistant strains are now widely disseminated throughout the United States. These drugs are no longer recommended for treatment of gonorrhea; cephalosporins are the only drugs currently recommended. Most of the treatment failures resulting from the use of oral cephalosporins have been reported in Asian countries. To ensure appropriate antibiotic therapy, physicians should ask patients who test positive for gonorrhea about recent travel to and sexual activity in this region.
MYCOPLASMA GENITALIUM INFECTION
Urethritis is characterized by urethral inflammation and can result from infectious and noninfectious conditions. Although N. gonorrhoeae and C. trachomatis are well established as clinically important infectious causes of urethritis, Mycoplasma genitalium is also associated with this condition. M. genitalium, which seems to be sexually transmitted, accounts for 15 to 25 percent of cases of nongonococcal urethritis in the United States. Treatment should be initiated as soon as possible after diagnosis. Although azithromycin and doxycycline are highly effective for chlamydial urethritis, M. genitalium infections respond better to azithromycin. Limited data indicate that M. genitalium also may have a role in persistent cervicitis. However, further research is needed.
LGV PROCTITIS/PROCTOCOLITIS
Sexually transmitted gastrointestinal syndromes include proctitis, proctocolitis, and enteritis. C. trachomatis (including lymphogranuloma venereum [LGV] serovars) is among the most common sexually transmitted pathogens involved. Patients with suspected or documented herpes proctitis should be treated in the same manner as those with genital herpes. If painful perianal ulcers are present, or if mucosal ulcers are detected on anoscopy, presumptive therapy should include a regimen for genital herpes and for LGV. Appropriate diagnostic testing for LGV should be performed in accordance with state and federal guidelines, and 100 mg of doxycycline should be given twice per day for three weeks. Among men who have sex with men, treatment of LGV proctitis/proctocolitis can be considered in those with anorectal chlamydia and either proctitis or human immunodeficiency virus infection.
SYPHILIS
Clinical signs of neurosyphilis include cranial nerve dysfunction, meningitis, stroke, altered mental status, loss of vibration sense, and auditory or ophthalmic abnormalities. Cerebrospinal fluid (CSF) abnormalities are common in persons with early syphilis. The Venereal Disease Research Laboratory in CSF (CSF-VDRL) is the standard serologic test for CSF. When reactive in the absence of substantial contamination of CSF with blood, it is considered diagnostic for neurosyphilis. Most other tests are insensitive and nonspecific, and must be interpreted relative to other test results and the clinical assessment. Therefore, the laboratory diagnosis of neurosyphilis usually depends on various combinations of reactive serologic test results, CSF cell count or protein, and a reactive CSF-VDRL, with or without clinical manifestations.
Early syphilis can be treated with a single 2-g dose of azithromycin. However, azithromycin resistance and treatment failure resulting from Treponema pallidum chromosomal mutations have been documented in the United States. Therefore, azithromycin should be used with caution only when treatment with penicillin or doxycycline is not feasible. Azithromycin should not be used in men who have sex with men or in pregnant women, and close follow-up is essential in persons using any alternative therapies.
HEPATITIS C
Sexual transmission of hepatitis C virus can occur, especially among persons infected with human immunodeficiency virus. Studies have found that among heterosexual persons and men who have sex with men, the risk of transmission increases with the number of sex partners. Group sex and the use of cocaine and other noninjection drugs during sex are associated with increased risk.
STD Evaluation After Sexual Assault
Trichomoniasis, bacterial vaginosis, gonorrhea, and chlamydia are the most commonly diagnosed STDs among women who have been sexually assaulted. Chlamydia and gonorrhea are of particular concern because of the risk of ascending infection.
Examinations of survivors of sexual assault should be conducted in a way that minimizes further trauma. The decision to obtain genital or other specimens for STD diagnosis should be made on an individual basis. An initial examination may include the following procedures:
Nucleic acid amplification testing for C. trachomatis and N. gonorrhoeae. These tests are preferred for the diagnostic evaluation of sexual assault victims, regardless of the sites of penetration.
Wet mount and culture or point-of-care testing of a vaginal swab specimen for T. vaginalis infection. The wet mount should also be examined for evidence of bacterial vaginosis and candidiasis.
Serum sample for immediate evaluation for human immunodeficiency virus infection, hepatitis B, and syphilis. Decisions to perform these tests should be made on an individual basis.
After the initial examination, follow-up examinations are necessary within one to two weeks to detect new infections acquired during or after the assault; to complete the hepatitis B vaccine series, if indicated; to complete counseling and treatment for other STDs; and to monitor adverse effects of and adherence to postexposure prophylactic regimens. Serologic tests for syphilis and human immunodeficiency virus infection can be repeated six weeks, three months, and six months after the assault if initial results were negative.
The following prophylactic regimen is recommended in survivors of sexual assault:
Postexposure hepatitis B vaccination, without hepatitis B immune globulin. This vaccine should be administered at the time of initial examination if the victim has not been vaccinated previously. Follow-up doses should be administered one to two and six months after the initial dose.
An empiric antimicrobial regimen for chlamydia, gonorrhea, and trichomoniasis.
Emergency contraception, if indicated.
