
Am Fam Physician. 2011;84(2):183-190
A more recent article on type 2 diabetes mellitus is available.
Author disclosure: No relevant financial affiliations to disclose.
Insulin therapy is recommended for patients with type 2 diabetes mellitus and an initial A1C level greater than 9 percent, or if diabetes is uncontrolled despite optimal oral glycemic therapy. Insulin therapy may be initiated as augmentation, starting at 0.3 unit per kg, or as replacement, starting at 0.6 to 1.0 unit per kg. When using replacement therapy, 50 percent of the total daily insulin dose is given as basal, and 50 percent as bolus, divided up before breakfast, lunch, and dinner. Augmentation therapy can include basal or bolus insulin. Replacement therapy includes basal-bolus insulin and correction or premixed insulin. Glucose control, adverse effects, cost, adherence, and quality of life need to be considered when choosing therapy. Metformin should be continued if possible because it is proven to reduce all-cause mortality and cardiovascular events in overweight patients with diabetes. In a study comparing premixed, bolus, and basal insulin, hypoglycemia was more common with premixed and bolus insulin, and weight gain was more common with bolus insulin. Titration of insulin over time is critical to improving glycemic control and preventing diabetes-related complications.
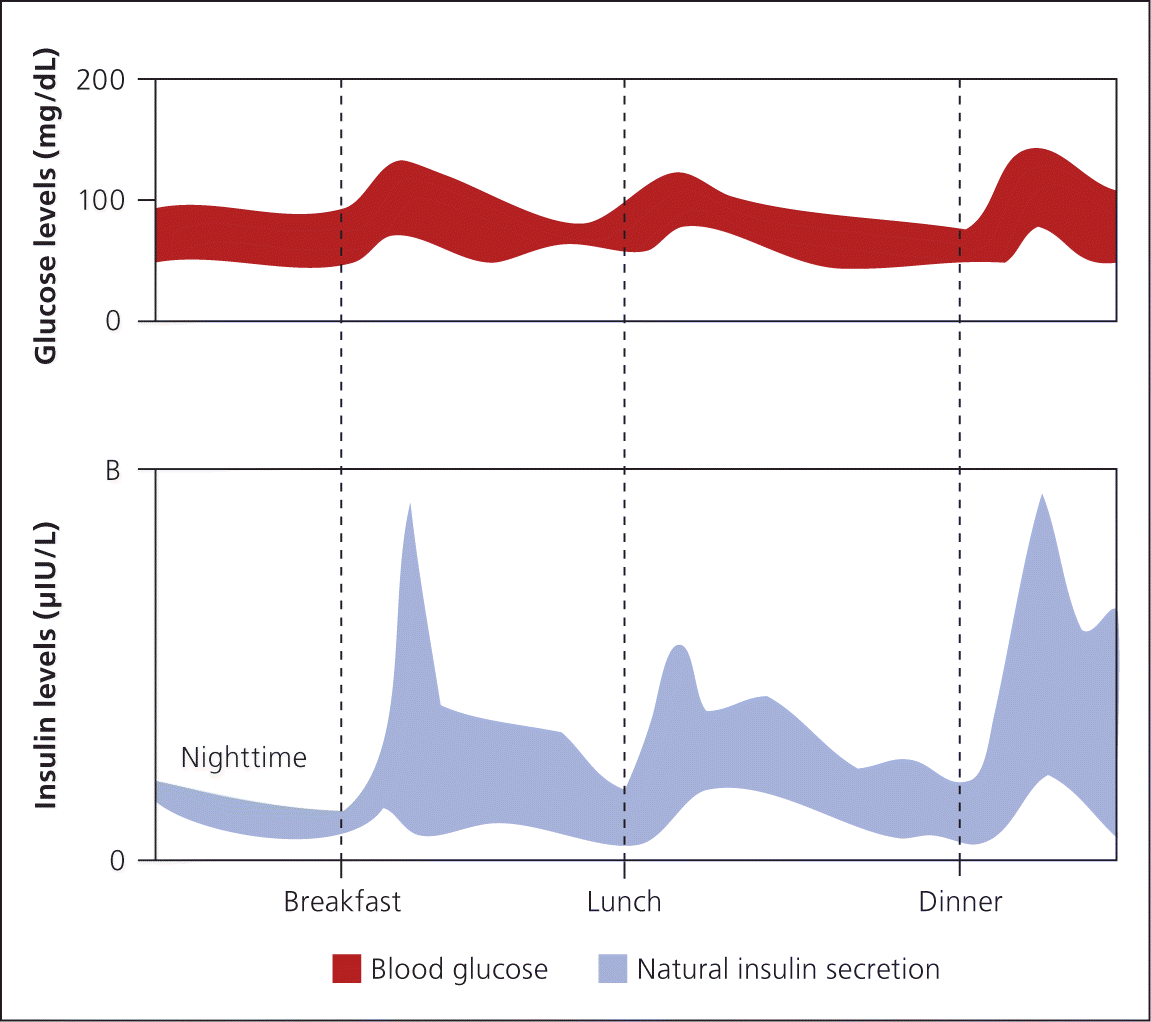
Insulin is secreted continuously by beta cells in a glucose-dependent manner throughout the day. It is also secreted in response to oral carbohydrate loads, including a large first-phase insulin release that suppresses hepatic glucose production followed by a slower second-phase insulin release that covers ingested carbohydrates 1 (Figure 12 ).
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Analogue insulin is as effective as human insulin but is associated with less postprandial hyperglycemia and delayed hypoglycemia. | A | 17–19 |
| Fasting glucose readings should be used to titrate basal insulin, whereas both preprandial and postprandial glucose readings should be used to titrate mealtime insulin. | C | 1 |
| Lipohypertrophy due to repeated injections of insulin in the same area leads to poor insulin absorption and may cause early postprandial hyperglycemia and/or delayed hypoglycemia. | C | 35 |
| Metformin (Glucophage) combined with insulin is associated with decreased weight gain, a lower insulin dose, and less hypoglycemia compared with insulin alone. | B | 38 |
| Oral medications should not be abruptly discontinued when starting insulin therapy because of the risk of rebound hyperglycemia. | C | 40 |
Type 2 diabetes mellitus is associated with insulin resistance and slowly progressive beta-cell failure. By the time type 2 diabetes is diagnosed in patients, up to one-half of their beta cells are not functioning properly. 3 Beta-cell failure continues at a rate of about 4 percent each year. 4 Therefore, patients with type 2 diabetes often benefit from insulin therapy at some point after diagnosis.
Concerns About Insulin Therapy
Pain, weight gain, and hypoglycemia may occur with insulin therapy. Pain is associated with injection therapy and glucose monitoring, although thinner and shorter needles are now available to help decrease pain. Weight gain associated with insulin therapy is due to the anabolic effects of insulin, increased appetite, defensive eating from hypoglycemia, and increased caloric retention related to decreased glycosuria. In the U.K. Prospective Diabetes Study, patients with type 2 diabetes who were taking insulin gained an average of 8 lb, 13 oz (4 kg), which was associated with a 0.9 percent decrease in A1C level compared with patients on conventional therapy.5
Hypoglycemia may occur from a mismatch between insulin and carbohydrate intake, exercise, or alcohol consumption. Hypoglycemia has been associated with an increased risk of dementia and may have implications in cardiac arrhythmia. 6,7 All patients should be instructed on the symptoms and treatment of hypoglycemia. American Diabetes Association (ADA) guidelines recommend that the blood glucose level be checked if hypoglycemia is suspected (glucose level lower than 70 mg per dL [3.89 mmol per L]), then treated with a fast-acting carbohydrate, such as juice or glucose tablets. The blood glucose level should be rechecked after 15 minutes to make sure it has normalized.8
An epidemiologic study has raised concern about cancer risk with glargine (Lantus) and other insulin therapies. 9 Glargine is theoretically more likely to cause cancer because of its high affinity for insulin-like growth factor I receptor. A consensus statement by the ADA indicates that this possible risk needs further research but should not be a limiting factor in treatment choice.10 Finally, it is important to note that there have been no randomized controlled trials demonstrating reduced all-cause mortality or cardiovascular events with insulin augmentation in patients with type 2 diabetes.
Initiating Appropriate Insulin Therapy
The American College of Endocrinology and the American Association of Clinical Endocrinologists recommend initiation of insulin therapy in patients with type 2 diabetes and an initial A1C level greater than 9 percent, or if the diabetes is uncontrolled despite optimal oral glycemic therapy. 11 Insulin may be used alone or in combination with oral medications, such as metformin (Glucophage). This recommendation is based on expert opinion, and not on the results of randomized controlled trials comparing different approaches in patients with an initial A1C level greater than 9 percent.
In the U.K. Prospective Diabetes Study, early intensive glucose control starting with a sulfonylurea, then metformin, then insulin was associated with a 25 percent reduction in microvascular complications and a 12 percent risk reduction in any diabetes-related end point, but was not associated with a reduction in all-cause mortality. 5 A subgroup of patients randomized to intensive therapy with metformin alone had a 36 percent reduction in all-cause mortality. 12 This supports current ADA guidelines that recommend using metformin as first-line pharmacologic therapy; however, additional therapies need to be added if diabetes is not controlled with metformin alone.
Recent trials have shown that intensive glucose control (i.e., an A1C target of less than 6.0 or 6.5 percent) does not improve, and may worsen, clinical outcomes. 13–15 Older patients with a limited life expectancy and patients with a high risk of hypoglycemia, previous cardiovascular disease or advanced microvascular disease, longer diabetes duration, or multiple comorbid conditions may benefit from less stringent glucose control.16
Analogue Versus Human Insulin
Glucose control, adverse effects, cost, adherence, and quality of life need to be considered when choosing a type of insulin. In general, analogue insulin is similar to human insulin in controlling diabetes, although some trials have found higher mean A1C levels in patients taking analogue insulin compared with human insulin. 17 Analogue insulin usually causes less postprandial hyperglycemia and delayed hypoglycemia. 18,19 In a recent meta-analysis, glycemic control was not improved with analogue insulin compared with human insulin, but nocturnal hypoglycemia was reduced.17
An industry-funded cost-effectiveness analysis found that the increased cost of medication is more than off set by the reduction in hypoglycemic events. 20 However, the analysis assumed a cost differential of 14 percent, which is inconsistent with current pricing ($119 for a 10-mL vial of glargine insulin compared with $73 for a 10-mL vial of NPH insulin [Humulin N], a 63 percent difference). 20,21 Cost-effectiveness analyses have differed regarding the long-term cost savings of using analogue insulin in patients with type 2 diabetes, with industry-sponsored studies finding reduced cost22 and government-sponsored studies finding no cost reduction. 23 Measures of adherence and quality of life have been improved with analogue insulin compared with human insulin. 24,25
Choosing the Correct Type of Insulin
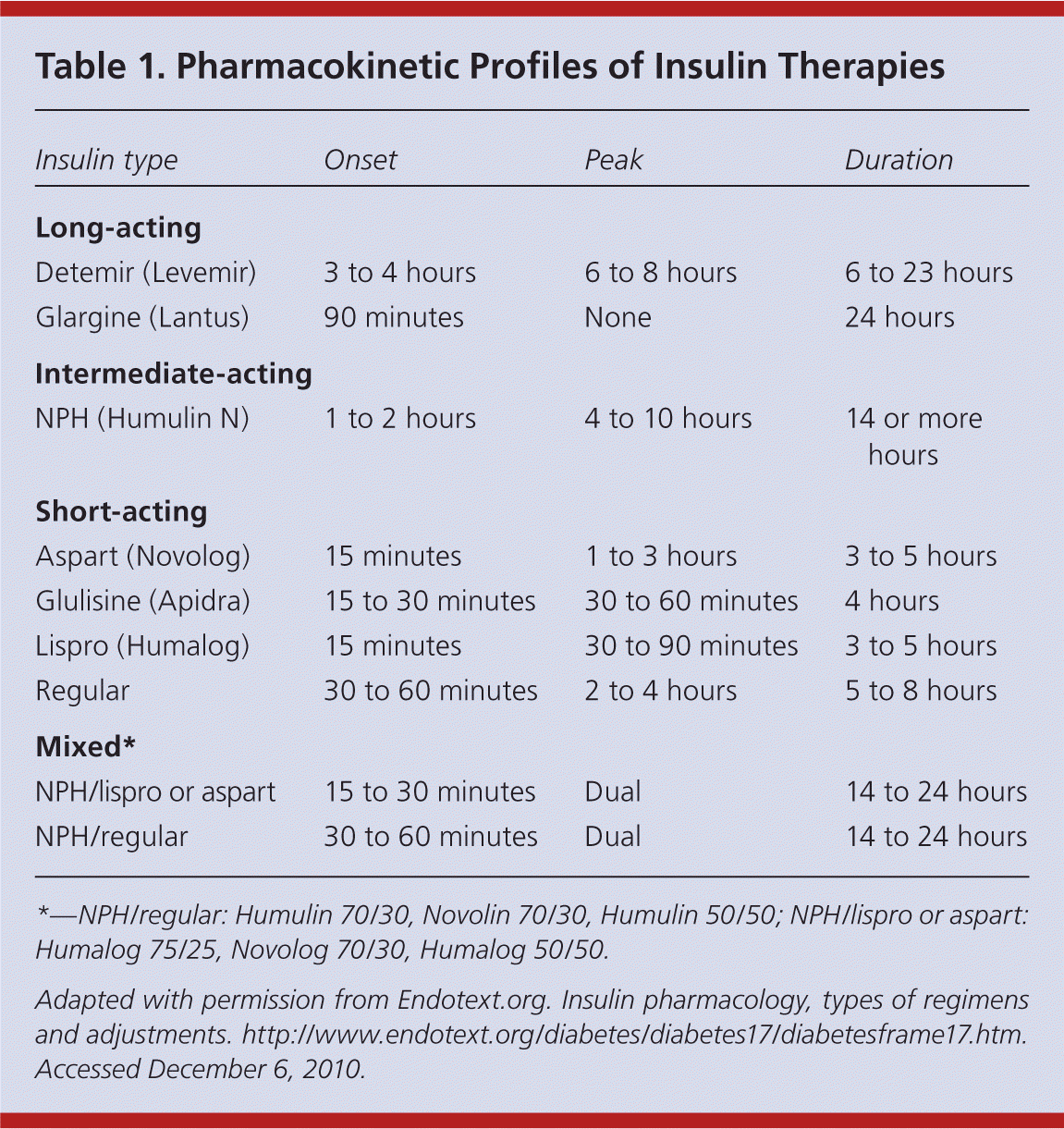
| Insulin type | Onset | Peak | Duration |
|---|---|---|---|
| Long-acting | |||
| Detemir (Levemir) | 3 to 4 hours | 6 to 8 hours | 6 to 23 hours |
| Glargine (Lantus) | 90 minutes | None | 24 hours |
| Intermediate-acting | |||
| NPH (Humulin N) | 1 to 2 hours | 4 to 10 hours | 14 or more hours |
| Short-acting | |||
| Aspart (Novolog) | 15 minutes | 1 to 3 hours | 3 to 5 hours |
| Glulisine (Apidra) | 15 to 30 minutes | 30 to 60 minutes | 4 hours |
| Lispro (Humalog) | 15 minutes | 30 to 90 minutes | 3 to 5 hours |
| Regular | 30 to 60 minutes | 2 to 4 hours | 5 to 8 hours |
| Mixed* | |||
| NPH/lispro or aspart | 15 to 30 minutes | Dual | 14 to 24 hours |
| NPH/regular | 30 to 60 minutes | Dual | 14 to 24 hours |
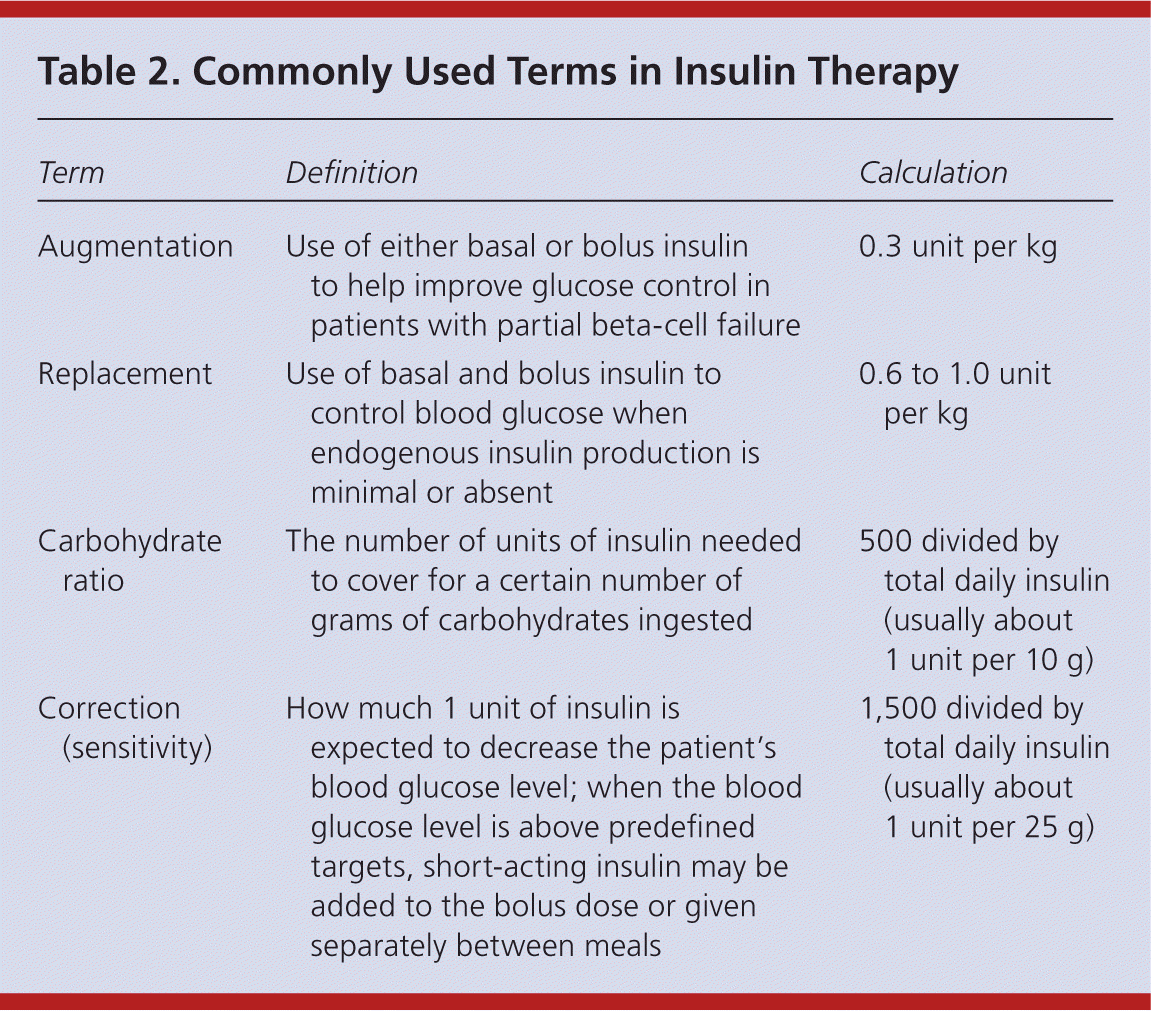
| Term | Definition | Calculation |
|---|---|---|
| Augmentation | Use of either basal or bolus insulin to help improve glucose control in patients with partial beta-cell failure | 0.3 unit per kg |
| Replacement | Use of basal and bolus insulin to control blood glucose when endogenous insulin production is minimal or absent | 0.6 to 1.0 unit per kg |
| Carbohydrate ratio | The number of units of insulin needed to cover for a certain number of grams of carbohydrates ingested | 500 divided by total daily insulin (usually about 1 unit per 10 g) |
| Correction (sensitivity) | How much 1 unit of insulin is expected to decrease the patient's blood glucose level; when the blood glucose level is above predefined targets, short-acting insulin may be added to the bolus dose or given separately between meals | 1,500 divided by total daily insulin (usually about 1 unit per 25 g) |
AUGMENTATION ONLY
In one study, patients who had uncontrolled type 2 diabetes and were taking a sulfonylurea and metformin were randomized to receive premixed, bolus, or basal analogue insulin. Median A1C levels were similar among the groups, but hypoglycemia was more common in the premixed and bolus groups, and weight gain was more common in the bolus group. 28 The results of this study suggest that adding basal insulin to oral antihyperglycemics is similarly effective but has fewer adverse effects compared with adding premixed or bolus insulin
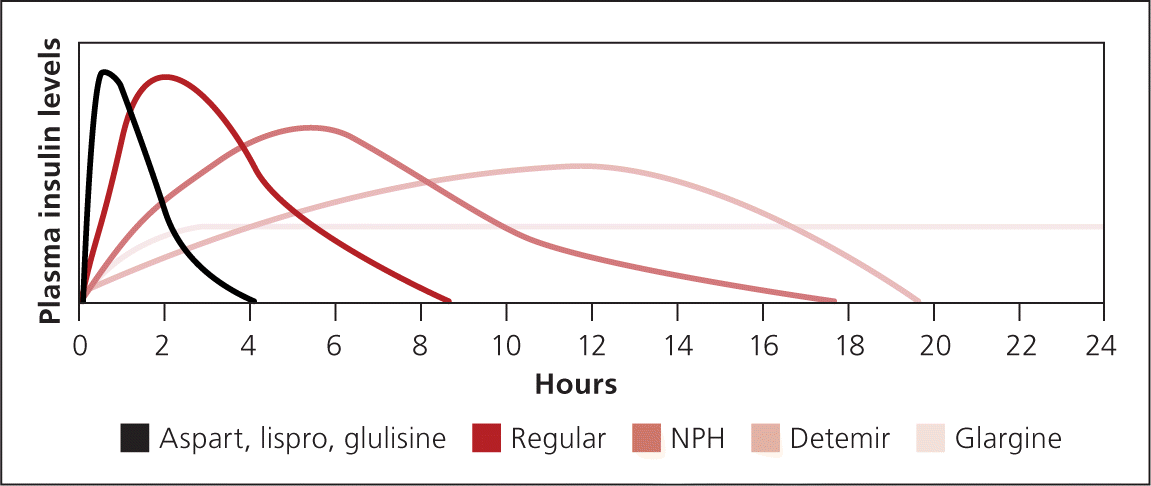
The goal of basal insulin is to suppress hepatic glucose production and improve fasting hyperglycemia (Figure 32 ). If basal insulin is titrated too high, it will also partially cover meals and lead to hypoglycemia during the night or if a meal is missed. Long-acting analogue insulin may be administered once or twice daily, depending on the dose. Lower doses may not last 24 hours, whereas higher doses may impede insulin absorption. NPH may be administered one to three times daily. NPH is often used during pregnancy and in patients who are unable to afford the up-front cost of analogue insulin.
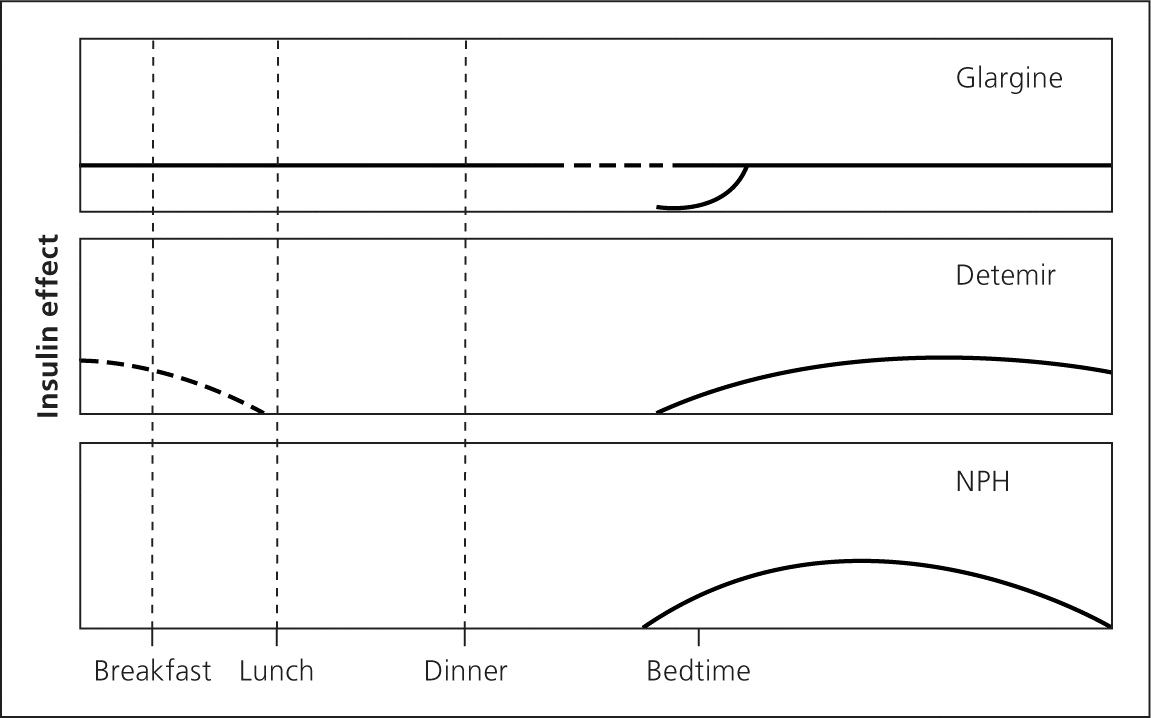
Bolus insulin may also be used for augmentation (Figure 42 ). Short-acting insulin is administered before meals to cover the carbohydrate load. Short-acting analogue insulin is given up to 15 minutes before a meal to maintain two-hour postprandial glucose levels. Taking insulin after meals increases the risk of early postprandial hyperglycemia followed by delayed hypoglycemia. 29 Regular insulin may be used instead and is given 30 to 45 minutes before meals.
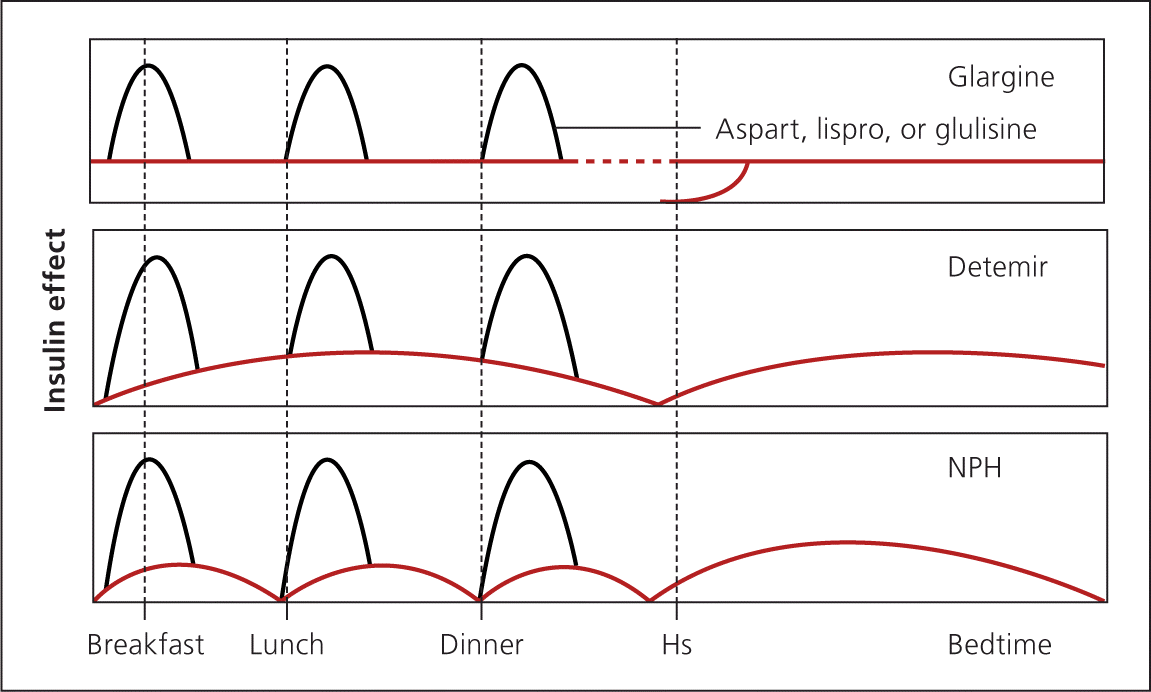
REPLACEMENT
Replacement therapy includes basal-bolus insulin and correction or premixed insulin; an insulin pump may be used, but is beyond the scope of this article. Replacement should be considered for patients with type 2 diabetes that is uncontrolled with augmentation therapy and who are able to comply with such a regimen or who desire tighter control. Bolus insulin should be added to basal insulin if fasting glucose goals are met but postprandial goals are not. When blood glucose levels are above predefined targets, additional short-acting insulin may be added to the bolus dose before meals. For example, a patient takes 40 units of glargine daily and 12 units of lispro (Humalog) before each meal, and has a correction factor of 1 unit for every 20 mg per dL (1.11 mmol per L) above 120 mg per dL (6.66 mmol per L). If the blood glucose level at breakfast is 160 mg per dL (8.88 mmol per L), the patient would take 12 units of lispro for the meal plus an additional 2 units for correction before eating.
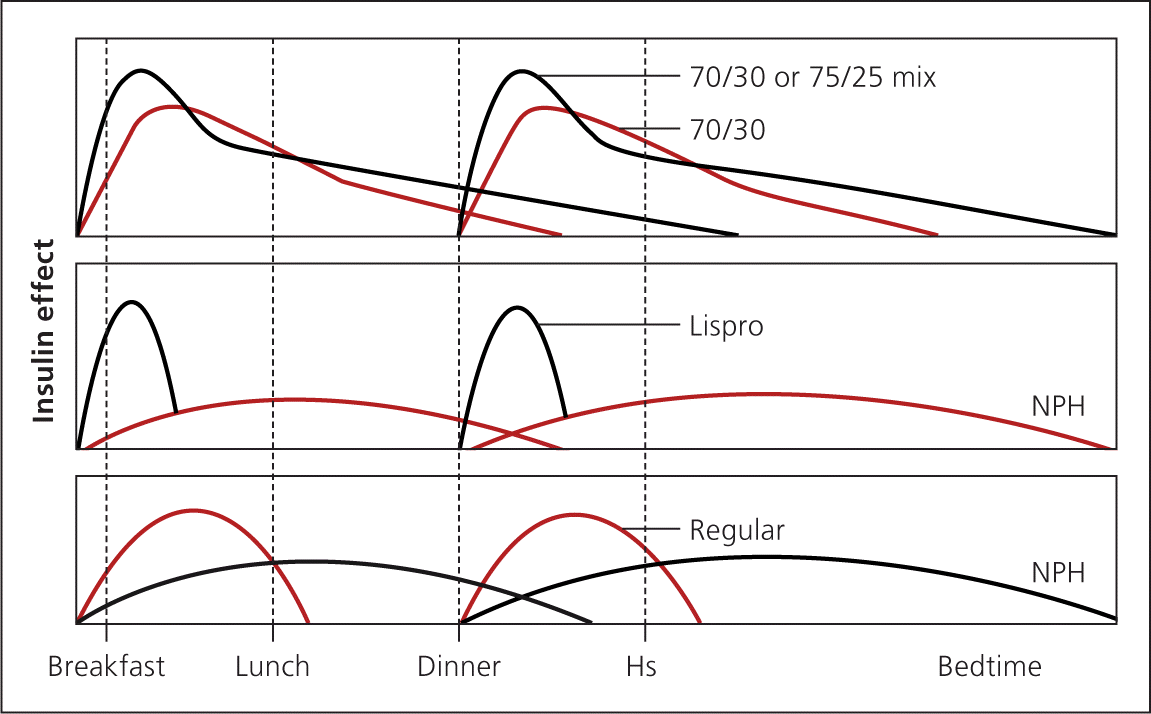
Premixed insulin similarly reduces A1C compared with basal-bolus insulin.30 NPH is combined with regular insulin or short-acting analogue insulin and is administered two or three times daily. Fewer injections are needed, but patients are more restricted in their eating habits and schedule. Patients must eat breakfast, lunch, dinner, and possibly midmorning and bedtime snacks to prevent hypoglycemia. If used, correction insulin must be administered separately with a short-acting insulin. This may increase the number of injections compared with basal-bolus therapy (Figure 52 ).
Initiation, Titration, and Follow-Up
The initial dosage of insulin is individualized based on the patient's insulin sensitivity. Insulin therapy may be started with a set dosage, such as 10 units of glargine daily, or by using weight-based equations. Equations to estimate augmentation, replacement, carbohydrate ratio, and correction therapy are listed in Table 2. When using replacement therapy, 50 percent of the total daily insulin dose is given as basal and 50 percent as bolus, divided up before breakfast, lunch, and dinner. For example, a 120-kg (265-lb) patient requiring basal-bolus and correction insulin would need 36 units of basal insulin (0.3 unit per kg); 12 units of short-acting insulin before each meal (0.3 unit per kg divided among three meals); and, for correction, 1 unit of a short-acting insulin for every 25 mg per dL (1.39 mmol per L) above the set glucose target.
Titration of insulin over time is critical to improving glycemic control and preventing diabetes-related complications. 5,31 Current ADA goals for glucose control are outlined in Table 3.16 Fasting glucose readings are used to titrate basal insulin, whereas both preprandial and postprandial glucose readings are used to titrate mealtime insulin. 1 Physicians may increase or decrease basal and/or bolus insulin by 10 percent based on the patient's home glucose readings. Some physicians have adopted the Treat-to-Target Trial's titration schedule for basal insulin (Table 4).31 It is also safe and effective to give patients autonomy to adjust insulin on their own. 32 Typically, insulin is increased or decreased by 2 to 3 units every three to seven days if the patient's blood glucose level is not within set targets.
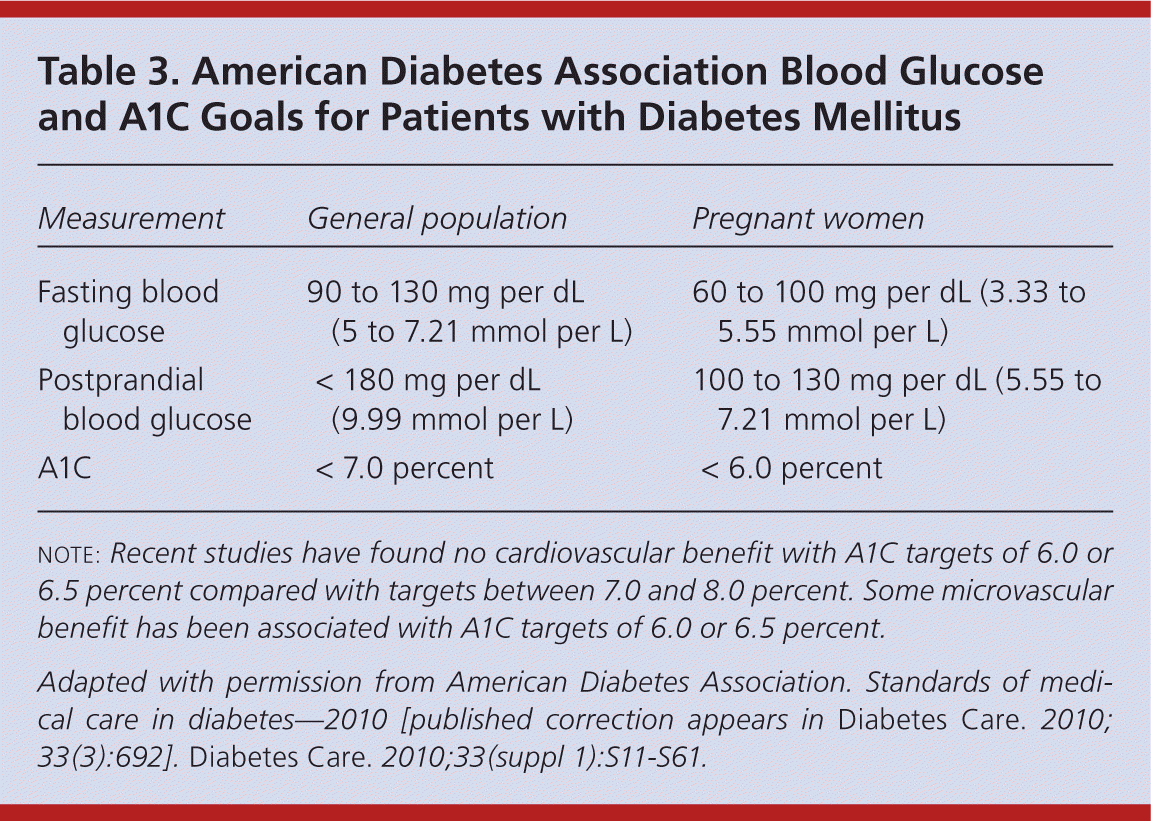
| Measurement | General population | Pregnant women |
|---|---|---|
| Fasting blood glucose | 90 to 130 mg per dL (5 to 7.21 mmol per L) | 60 to 100 mg per dL (3.33 to 5.55 mmol per L) |
| Postprandial blood glucose | < 180 mg per dL (9.99 mmol per L) | 100 to 130 mg per dL (5.55 to 7.21 mmol per L) |
| A1C | < 7.0 percent | < 6.0 percent |
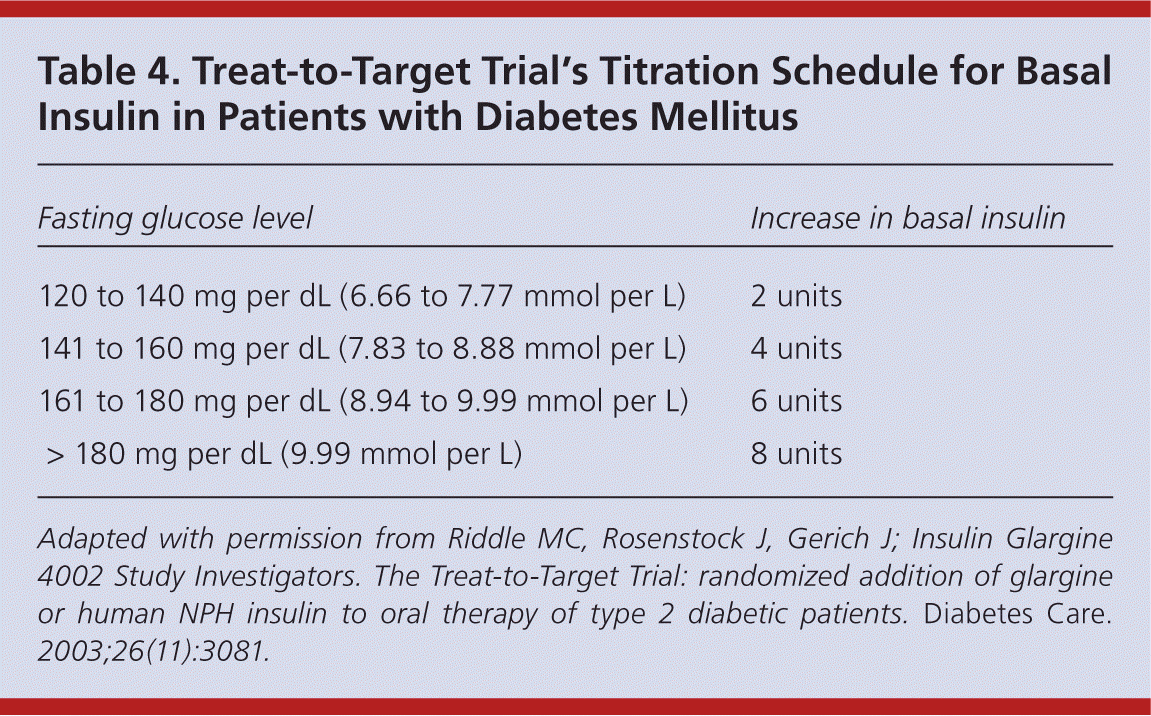
| Fasting glucose level | Increase in basal insulin |
|---|---|
| 120 to 140 mg per dL (6.66 to 7.77 mmol per L) | 2 units |
| 141 to 160 mg per dL (7.83 to 8.88 mmol per L) | 4 units |
| 161 to 180 mg per dL (8.94 to 9.99 mmol per L) | 6 units |
| > 180 mg per dL (9.99 mmol per L) | 8 units |
Insulin Injection Technique
Insulin is effective only if administered appropriately. Injections may be given in the abdomen, outer thigh, back of the arm, and flank/buttocks region. The needle should be placed at a 90-degree angle to the skin and held in place for five to 10 seconds after injection to prevent insulin leakage. 8
Rotation of injection sites is important to prevent lipohypertrophy (i.e., scar tissue from repeated injections in the same area). Lipohypertrophy leads to poor insulin absorption and depot formation, which may randomly release insulin, causing early postprandial hyperglycemia and/or delayed hypoglycemia. 35
Insulin is available in pens and vials. Benefits of insulin pens include the convenience of storing at room temperature for 28 days after opening and ease of use for patients with visual or dexterity problems. Patients with visual difficulties may listen to the “clicks” of the insulin pen to count the number of units. Patients should be instructed to prime the insulin pen before every use. Priming consists of drawing up 1 or 2 units of insulin and injecting into the air to allow the insulin to fill the needle.
Using Insulin with Oral Medications
Many oral medications are safe and effective when combined with insulin therapy. To maximize benefit without causing significant adverse effects, it is important to consider the mechanism of action for different therapies.
Insulin sensitizers have been proven safe and effective when combined with insulin therapy. 36,37 Metformin is usually continued indefinitely after the patient starts insulin therapy because it reduces cardiovascular risk in overweight patients with type 2 diabetes.12 Metformin combined with insulin is also associated with decreased weight gain, a lower insulin dosage, and less hypoglycemia compared with insulin alone. 38 Thiazolidinediones improve insulin sensitivity but may increase weight gain, fluid retention, and risk of congestive heart failure when combined with insulin.36 Thiazolidinediones also have not been shown to reduce macrovascular complications or all-cause mortality.
Alpha-glucosidase inhibitors delay absorption of carbohydrates in the gastrointestinal tract to decrease postprandial hyperglycemia. These medications are safe and effective when combined with insulin.39
Insulin secretagogues (sulfonylureas and glitinides) can be combined with insulin, especially when only basal augmentation is being used. However, there is a possible increased risk of hypoglycemia that needs to be monitored closely. Usually by the time insulin is required for meals, insulin secretagogues are not effective or necessary. However, it is recommended to continue oral medications while starting insulin to prevent rebound hyperglycemia.40 After the diabetes is controlled, the patient may be weaned off of oral medications.
Incretin therapies include dipeptidyl-peptidase IV inhibitors (sitagliptin [Januvia] and saxagliptin [Onglyza]) and glucagon-like peptide-1 agonists (exenatide [Byetta] and liraglutide [Victoza]). Sitagliptin is currently the only one of these medications that is approved by the U.S. Food and Drug Administration for combination therapy with insulin. This combination is associated with improved fasting and postprandial glucose control.41 Exenatide combined with insulin has been associated with improved glycemic control, weight loss, and no increased risk of hyperglycemia.42 As with thiazolidinediones, glucagon-like peptide-1 agonists and saxagliptin have not been shown to reduce macrovascular events or all-cause mortality.
Data Sources: A PubMed search was completed in Clinical Queries using the key terms intensive insulin therapy, insulin and cancer, insulin and weight gain, UKPDS, self-titration insulin, human and analog insulin, metformin and insulin, sulfonylurea and insulin, and incretin and insulin. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. Search dates: August 24, 2010, and November 29, 2010.
