
Am Fam Physician. 2012;85(11):1086-1093
Patient information: See related handout on Lyme disease, written by the authors of this article.
Author disclosure: No relevant financial affiliations to disclose.
Lyme disease, caused by the bacterium Borrelia burgdorferi, is the most common tick-borne illness in the United States. Transmission occurs primarily through the bite of an infected deer tick (Ixodes scapularis). Identification of an erythema migrans rash following a tick bite is the only clinical manifestation sufficient to make the diagnosis of Lyme disease in the absence of laboratory confirmation. The Centers for Disease Control and Prevention recommends a two-tier serologic testing protocol using an enzyme-linked immunosorbent assay initially, followed by the more specific Western blot to confirm the diagnosis when the assay samples are positive or equivocal. The treatment of Lyme disease is determined mainly by the clinical manifestations of the disease. Doxycycline is often the preferred agent for oral treatment because of its activity against other tick-borne illnesses. Preventive measures include avoiding areas with high tick burdens, wearing protective clothing, using tick repellants (e.g., diethyltoluamide [DEET]), performing frequent body checks and bathing following outdoor activities, and instituting environmental landscape modifications (e.g., grass mowing, deer exclusion fencing) to reduce the tick burden. Although there is controversy regarding treatment of post–Lyme disease syndrome and chronic Lyme disease, there is no biologic or clinical trial evidence indicating that prolonged antibiotic therapy is of benefit.
Lyme disease is the most common tick-borne illness in the United States. It is caused by the bacterium Borrelia burgdorferi, and is transmitted primarily by the deer tick (Ixodes scapularis; Ixodes pacificus on the West Coast). Following its discovery in children and adults in Lyme, Conn., in 1977,1 its incidence has increased steadily in the United States.2
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Erythema migrans rash following a tick bite is the only clinical manifestation sufficient to make the diagnosis of Lyme disease in the absence of laboratory confirmation. | C | 12, 20 |
| For diagnosis of Lyme disease, the Centers for Disease Control and Prevention recommends a two-tier serologic testing protocol using an enzyme-linked immunosorbent assay initially, followed by the more specific Western blot to confirm the diagnosis when the assay samples are positive or equivocal. | C | 25 |
| Doxycycline is effective for the treatment of early Lyme disease and is the preferred agent for oral treatment because of its activity against other tick-borne illnesses. | C | 20 |
| Prolonged antibiotic therapy for chronic Lyme disease or post–Lyme disease syndrome, beyond the standard recommendations, provides no benefit and is not recommended. | C | 20, 21, 32 |
| Recommended measures to prevent Lyme disease include avoiding areas with high tick burdens, wearing protective clothing, using tick repellants (e.g., diethyltoluamide [DEET]), performing frequent body checks for ticks and bathing following outdoor activities, and instituting environmental landscape modifications (e.g., grass mowing, deer exclusion fencing, removing leaf litters and woodpiles) to reduce the tick burden. | C | 3, 4, 39, 40 |
| Antimicrobial prophylaxis with a single 200-mg dose of doxycycline is recommended for adults with exposure to Ixodes scapularis if prophylaxis can be given within 72 hours of tick removal and there is at least a 20 percent rate of tick infection with Borrelia burgdorferi in the area. Children eight years or older may also be given a single 4-mg-per-kg dose of doxycycline (maximal dose of 200 mg) for prophylaxis. | B | 3, 4, 20, 42 |
Epidemiology
Between 1992 and 2006, the number of cases of Lyme disease reported to the Centers for Disease Control and Prevention (CDC) increased from 9,908 cases per year to 19,931 cases per year.2 However, true incidence data may be affected by reliance on voluntary case reporting, greater awareness of the condition, and misdiagnosis. The risk of Lyme disease is greatest in the following states: Connecticut, Delaware, Maine, Maryland, Massachusetts, Minnesota, New Hampshire, New Jersey, New York, Pennsylvania, Rhode Island, and Wisconsin.2 Lyme disease can occur in both sexes and at any age; however, it occurs primarily in males, and the peak ages of incidence are five to nine years and 55 to 59 years.2–4
Transmission
A recent study found that 34 percent of sampled I. scapularis in northern New Jersey were infected with B. burgdorferi.5 Although nymphs and adult ticks can transmit the organism, it is more likely with the former. Infection occurs primarily during the late spring and summer months when nymphs are most active and persons spend the most time outdoors, but cases have been reported throughout the year.2
The duration of tick attachment is a critical factor affecting the risk of transmission.6–8 After attachment, the tick feeds and becomes engorged, discharging its saliva into the bite wound. It takes 36 to 48 hours after attachment for B. burgdorferi to migrate from the midgut of the tick to the salivary glands.3,4,9 How long the tick is attached (usually at least 36 hours) and whether it is engorged are two of the most important factors to consider when assessing the risk of transmission.10
Clinical Manifestations
Symptoms of early Lyme disease usually begin one to two weeks after a tick bite (range of three to 30 days).4,11,12 There are three well-recognized clinical stages of Lyme disease, and clinical manifestations are different at each stage (Table 1).3,4,12,13 As many as 80 percent of patients develop the characteristic erythema migrans rash (Figure 112 ), which may be confused with other similar conditions2,4,11,12 (Table 211,14 ). Erythema migrans is classically reported as a single lesion, and most commonly appears as a uniform erythematous oval to circular rash with a median diameter of 16 cm (range of 5 to 70 cm). Approximately 19 percent of erythema migrans rashes are a “bull's-eye” rash.11,14 Multiple erythema migrans lesions may occur in up to 10 to 20 percent of patients.11,14 Associated symptoms are similar to a nonspecific viral illness and often include fatigue, malaise, fever, chills, myalgia, and headache. Following this initial stage, the bacteria disseminate systemically via the lymphatic system or blood.3,15 With untreated disease, the most common sites of extracutaneous involvement are the joints, nervous system, and cardiovascular system.2,11
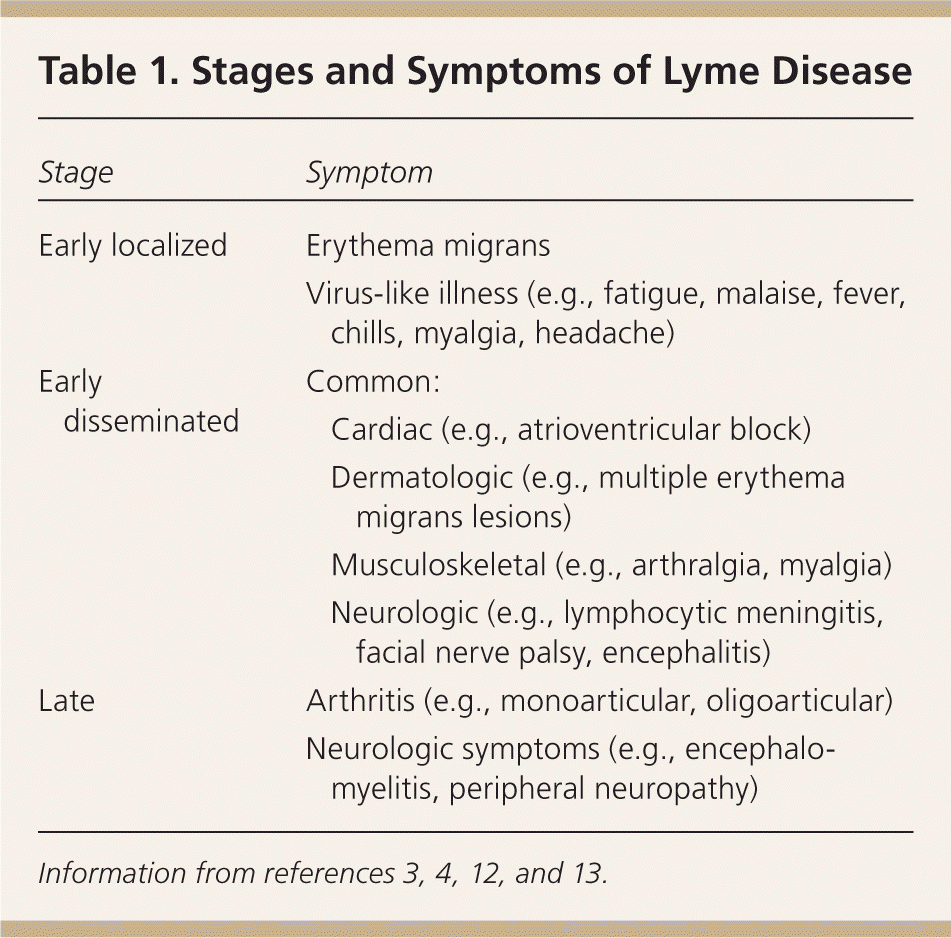
| Stage | Symptom | |
|---|---|---|
| Early localized | Erythema migrans | |
| Virus-like illness (e.g., fatigue, malaise, fever, chills, myalgia, headache) | ||
| Early disseminated | Common: | |
| Cardiac (e.g., atrioventricular block) | ||
| Dermatologic (e.g., multiple erythema migrans lesions) | ||
| Musculoskeletal (e.g., arthralgia, myalgia) | ||
| Neurologic (e.g., lymphocytic meningitis, facial nerve palsy, encephalitis) | ||
| Late | Arthritis (e.g., monoarticular, oligoarticular) | |
| Neurologic symptoms (e.g., encephalomyelitis, peripheral neuropathy) | ||


| Condition | Morphology/size | Location |
|---|---|---|
| Cellulitis | Homogenous erythematous lesions associated with edema, tenderness, and warmth | Most commonly adjacent to an ulcer, laceration, or wound |
| Erythema multiforme | Symmetric lesions (most are less than 2 cm in diameter) with central clearing | Diffuse lesions with mucous membrane involvement |
| Granuloma annulare | Scaling erythematous lesions with central clearing | Most commonly located on the feet and hands |
| Hypersensitivity reaction/dermatitis | Lesions of variable shape and size that may resemble certain objects (e.g., tape, nickel coins, necklaces) | Variable |
| Methicillin-resistant Staphylococcus aureus infection | Erythematous lesions of variable size that may be associated with necrotic eschar | Most commonly located on the extremities, but may involve the axilla, abdomen, chest, or back |
| Nummular eczema | Coin-shaped erythematous lesions that range in size from 2 to 10 cm | Most commonly located on the back and hands |
| Spider bite | Erythematous lesions of variable size that may be associated with necrotic eschar | Most commonly located on the extremities |
| Tinea (ringworm) | Annular or ring-form lesions of variable size with central clearing | Variable |
| Urticaria | Raised erythematous lesions with an associated serpiginous border | Variable |
Musculoskeletal symptoms are the most common extracutaneous manifestations of disseminated disease and can occur with early or late disease.3,16,17 Common manifestations of early disease include transient oligoarticular symptoms of arthralgia or myalgia that may include joint swelling.3,4,12,16,17 Arthritis is usually a manifestation of late disease, and occurs in up to 60 percent of untreated patients. Patients typically present approximately six months after infection with joint pain and swelling, and synovial fluid findings that suggest an inflammatory process.16 Chronic arthritis primarily involves the knees and hips.
Neurologic involvement, affecting up to 15 percent of untreated patients, can include lymphocytic meningitis, cranial neuropathies (primarily unilateral, but rarely bilateral, facial nerve palsy), motor or sensory radiculoneuropathy, mononeuritis multiplex, cerebellar ataxia, and myelitis.3,17 Patients may present with altered mental status, headaches, and neck pain and stiffness.17 The classic triad of meningitis, cranial neuropathy, and radiculoneuropathy has been described, although these conditions do not always occur together. Lyme disease must be included in the differential diagnosis of a seventh cranial nerve (Bell) palsy in endemic areas. Rarely, late disease may present as a subtle, subacute encephalopathy or axonal polyneuropathy that usually manifests as altered mentation, cognitive impairment, insomnia, or personality changes.3,16,17
Cardiac involvement usually occurs within one to two months after infection (range of less than one week to seven months).17–19 Lyme carditis is a less common complication of systemic disease, occurring in approximately 4 to 10 percent of patients.19 It may present as chest pain, dyspnea on exertion, fatigue, palpitations, or syncope, and often includes some form of atrioventricular block.4,12,18,19 A study of 105 patients with Lyme carditis reported that 49 percent had third-degree atrioventricular block, 28 percent had some form of second- or first-degree atrioventricular block, and 23 percent had no conduction abnormalities.18 Other less common manifestations include myopericarditis, bundle branch block, and heart failure.17–19
Lyme disease can progress to a late stage months to years after the initial tick bite, which may result in substantial morbidity, primarily from chronic arthritis.3,16,20,21 These complications are distinct from what have recently been named “post–Lyme disease syndrome,” which refers to the nonspecific symptoms (e.g., fatigue) that may persist after successful treatment, and “chronic Lyme disease,” which refers more broadly to chronic symptoms occurring in patients who may or may not have Lyme disease.20,21
Diagnosis
HISTORY AND PHYSICAL EXAMINATION
The clinical diagnosis of Lyme disease can be straightforward in patients with a history of tick exposure and the characteristic finding of an erythema migrans rash.12 The CDC has defined erythema migrans rash as an expanding red macule or papule that must reach at least 5 cm in size (with or without central clearing).11 According to the Infectious Diseases Society of America (IDSA) guidelines, erythema migrans rash is the only clinical manifestation sufficient to make the diagnosis of Lyme disease in the absence of laboratory confirmation.20 Although one study concluded that primary care physicians in a Lyme disease–endemic area of France correctly identified erythema migrans in 72 percent of patients,22 the study was limited by lack of complete clinical information for the participants.
A number of conditions resemble erythema migrans (Table 211,14 ); however, the rapid and prolonged expansion of an erythematous lesion is unique to erythema migrans.11 Lesions most often occur at anatomic sites that are unusual for cellulitis and other conditions that mimic erythema migrans (e.g., back, groin, abdomen, axilla, popliteal fossa); therefore, a complete skin examination should be performed before excluding erythema migrans.20
DIAGNOSTIC TESTING
Direct and indirect approaches have been used in the laboratory to assist in the diagnosis of Lyme disease. Direct methods involve culture or techniques that detect B. burgdorferi–specific proteins or nucleic acids, whereas indirect methods involve serology to detect antibodies.4 Although culture remains the diagnostic standard, it is not routinely available and its usefulness has been limited to skin biopsy samples in patients with a single early erythema migrans lesion or plasma in patients with multiple erythema migrans lesions.23,24
The CDC and IDSA recommend serology as the preferred initial diagnostic test.20,25 Currently, the CDC recommends a two-tier protocol using an enzyme-linked immunosorbent assay initially, followed by the more specific Western blot to confirm the diagnosis when the assay samples are positive or equivocal.25 The accuracy of a two-tier protocol in an endemic area was recently demonstrated (Table 3); however, the study may have overestimated the accuracy of this approach because of its case-control design.26

| Stage of Lyme disease | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) |
|---|---|---|---|---|
| Early localized | ||||
| Acute phase | 17 | 98 | 75 | 26 |
| Convalescent phase | 53 | 98 | 90 | 83 |
| Early disseminated | ||||
| Cardiac or neurologic manifestations | 100 | 98 | 87 | 100 |
| Multiple erythema migrans lesions | 43 | 98 | 89 | 79 |
| Late | ||||
| Arthritis or neurologic manifestations | 100 | 98 | 94 | 100 |
Polymerase chain reaction testing has the highest sensitivity for Lyme disease in synovial fluid samples from patients with untreated late Lyme arthritis.23,27,28 The IDSA recommends polymerase chain reaction testing as an option for selected patients with late Lyme arthritis or neurologic Lyme disease, but prefers testing for the presence of intrathecal antibody production with cerebrospinal fluid samples.20 The Lyme urine antigen test has a high false-positive rate and is generally not recommended.3,29
Physicians should understand reasons for false-positive and false-negative results with serology and the limitations inherent in this testing that often lead to an erroneous diagnosis and unnecessary antimicrobial treatment.4,12 Immunoglobulin M (IgM) and IgG produced in response to B. burgdorferi may persist for years following standard antimicrobial therapy; these persistently elevated levels are not an indication of ineffective treatment or chronic infection.4,30 Repeated serologic testing for documentation of treatment effectiveness or cure is not recommended in current guidelines.4,20,30 A new IgG enzyme-linked immunosorbent assay called the C6 peptide assay has comparable sensitivity and specificity to the standard two-tier protocol, but further evaluation is needed to determine the optimal use of this test.26, 31
Treatment
The treatment of Lyme disease is determined mainly by the clinical manifestations of the disease.20,21 In general, oral regimens are recommended for early localized disease. Intravenous regimens are reserved for patients with neurologic symptoms (e.g., meningitis), symptomatic cardiac disease, or, in a few cases, refractory Lyme arthritis. Many experts recommend doxycycline as the preferred agent for oral treatment because of its activity against other tick-borne illnesses (e.g., human granulocytic anaplasmosis), which can occur in up to 10 percent of patients with Lyme disease.20 Table 4 lists treatment options for Lyme disease.20

| Clinical symptoms | Treatment | ||||||
|---|---|---|---|---|---|---|---|
| Adults | Children | Duration | Comments | ||||
| Early localized | |||||||
| Doxycycline, 100 mg orally twice per day | Doxycycline, 4 mg per kg orally per day in two divided doses (maximum of 100 mg twice per day) in children eight years or older | 14 days (range of seven to 10 days for azithromycin; 10 to 21 days for doxycycline; and14 to 21 days for amoxicillin or cefuroxime axetil) |
| |||
| or | or | ||||||
| Amoxicillin, 500 mg orally three times per day | Amoxicillin, 50 mg per kg orally per day in three divided doses (maximum of 500 mg per dose) | ||||||
| or | or | ||||||
| Cefuroxime axetil (Ceftin), 500 mg orally twice per day | Cefuroxime axetil, 30 mg per kg orally per day in two divided doses (maximum of 500 mg per dose) | ||||||
| or | or | ||||||
| Azithromycin (Zithromax), 500 mg orally once per day | Azithromycin, 10 mg per kg orally per day (maximum of 500 mg per day) | ||||||
| Early disseminated | |||||||
| Ceftriaxone (Rocephin), 2 g intravenously per day | Ceftriaxone, 50 to 75 mg per kg intravenously per day (maximum of 2 g per dose) | 14 days (range of 14 to 21 days for ceftriaxone or cefotaxime and 10 to 28 days for oral doxycycline) |
| |||
| or | or | ||||||
| Cefotaxime (Claforan), 2 g intravenously every eight hours | Cefotaxime, 150 to 200 mg per kg intravenously per day in three or four divided doses (maximum of 6 g per day) | ||||||
| or | or | ||||||
| Doxycycline, 200 to 400 mg orally in two divided doses per day | Doxycycline, 4 to 8 mg per kg intravenously in two divided doses per day in children eight years or older (maximum of 100 to200 mg per dose) | ||||||
| Late | |||||||
| Same oral antibiotics as used for erythema migrans | Same oral antibiotics as used for erythema migrans | 28 days |
| |||
| Same intravenous antibiotics as used for neurologic symptoms of early disseminated disease | Same intravenous antibiotics as used for neurologic symptoms of early disseminated disease | 14 to 28 days | ||||
Some persons have advocated use of the term chronic Lyme disease (Table 5) to describe the persistence of nonspecific signs and symptoms in patients with or without clinical or laboratory evidence of Lyme disease.20,21 These advocates suggest that patients with the so-called post–Lyme disease syndrome (category 4) or antibiotic-refractory arthritis have a latent intracellular infection that may require months to years of antibiotic therapy for eradication.21 Critics of this position propose that the continued symptoms can be explained by an autoimmune response that is triggered by an association between Lyme disease and certain human leukocyte antigen haplotypes.16,17,21 Although controversy exists regarding post–Lyme disease syndrome and chronic Lyme disease treatment, four randomized clinical trials found no evidence that prolonged antibiotic therapy is of benefit.32–34 Therefore, the American Academy of Pediatrics, American Academy of Neurology, American College of Rheumatology, and IDSA do not recommend prolonged antibiotic therapy.20,21,35–37 A recent survey concluded that 97 percent of primary care physicians in Lyme disease–endemic areas did not diagnose or treat patients for chronic Lyme disease.38

| Category 1: Patients have nonspecific signs and symptoms suggestive of Lyme disease, but no objective clinical or laboratory evidence of infection |
| Category 2: Patients may have a history of tick exposure, with symptoms suggestive of Lyme disease, but have an alternative identifiable illness to explain symptoms |
| Category 3: Patients have serologic evidence suggestive of Lyme disease, but do not have clinical symptoms consistent with Lyme disease |
| Category 4: Patients have clinical and laboratory evidence of previous Lyme disease, but have persistent symptoms for longer than six months despite appropriate antibiotic therapy; also referred to as post–Lyme disease syndrome |
Prevention
Lyme disease prevention is an important part of management, particularly in those at high risk, such as park rangers and hikers. Avoiding areas with high tick burdens (e.g., wooded or grassy areas with a large deer population) is the best preventive measure.3,4,39,40 For persons living in endemic areas, additional recommended measures include wearing light-colored protective clothing, using tick repellants (e.g., diethyltoluamide [DEET]), performing frequent body checks for ticks and bathing following outdoor activities, and instituting environmental landscape modifications (e.g., grass mowing, deer exclusion fencing, removing leaf litters and woodpiles) to reduce the tick burden. A 2009 case-control study in an endemic area concluded that performing tick checks within 36 hours and bathing within two hours of spending time outside may reduce the risk of infection.40 Although a Lyme disease vaccine was approved in 1998, the manufacturer removed it from the market in 2002 because of poor sales.39
Removal of ticks within 24 hours of attachment can usually prevent acquisition of Lyme disease. Fine-tipped forceps are generally recommended for tick removal, with care taken to grasp the tick as close to the skin as possible without compressing the body.41 For engorged ticks or ticks that have been presumed to be attached for 36 hours or longer, antimicrobial prophylaxis with a single 200-mg dose of doxycycline is recommended for adults if it can be given within 72 hours of tick removal and there is at least a 20 percent rate of tick infection with B. burgdorferi in the area (e.g., any location within the top 12 endemic states).20,41 Children eight years or older also may be given a single dose of 4 mg per kg of doxycycline (maximal dose of 200 mg) for prophylaxis.
Because of lack of data and limited effectiveness of treatment, prophylaxis is not recommended in persons in whom doxycycline is contraindicated (e.g., pregnant women, children younger than eight years).3,4,20,42 Amoxicillin has not been recommended as a substitute for postexposure prophylaxis in patients with a contraindication to doxycycline, because a randomized controlled trial did not demonstrate a significant difference in the development of an erythema migrans lesion or in rate of seroconversion.20,39
Data Sources: A search of the medical literature was conducted using Medline (1995 to 2011) and EMBASE (1995 to 2011). Studies were identified using the terms Lyme disease and Lyme neuroborreliosis as either medical subject headings or free text, and with the following search terms as free text: Borrelia burgdorferi, Lyme carditis, Lyme arthritis, Lyme prevention, chronic Lyme disease, Lyme treatment, and post-Lyme syndrome. The search was restricted to articles published in English. Abstracts of the articles identified were assessed for appropriateness. All potential relevant articles were obtained and evaluated in detail. Bibliographies of all identified relevant studies were used to perform a recursive search of the medical literature for historically related articles. Also searched were the Cochrane database, National Guideline Clearinghouse database, Clinical Evidence, and Essential Evidence Plus. Search dates: April to June 2011, and November to December 2011.
