This is a corrected version of the article that appeared in print.
Am Fam Physician. 2012;86(7):661-667
A more recent article on community-acquired pneumonia in children is available.
Author disclosure: No relevant financial affiliations to disclose.
Community-acquired pneumonia is a potentially serious infection in children and often results in hospitalization. The diagnosis can be based on the history and physical examination results in children with fever plus respiratory signs and symptoms. Chest radiography and rapid viral testing may be helpful when the diagnosis is unclear. The most likely etiology depends on the age of the child. Viral and Streptococcus pneumoniae infections are most common in preschool-aged children, whereas Mycoplasma pneumoniae is common in older children. The decision to treat with antibiotics is challenging, especially with the increasing prevalence of viral and bacterial coinfections. Preschool-aged children with uncomplicated bacterial pneumonia should be treated with amoxicillin. Macrolides are first-line agents in older children. Immunization with the 13-valent pneumococcal conjugate vaccine is important in reducing the severity of childhood pneumococcal infections.
Community-acquired pneumonia (CAP) is a significant cause of respiratory morbidity and mortality in children, especially in developing countries.1 Worldwide, CAP is the leading cause of death in children younger than five years.2 Factors that increase the incidence and severity of pneumonia in children include prematurity, malnutrition, low socioeconomic status, exposure to tobacco smoke, and child care attendance.3
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| The absence of tachypnea is the most useful clinical finding for ruling out CAP in children. | C | 1, 15, 16 |
| Chest radiography has not been shown to improve clinical outcomes or change treatment of CAP in children. | B | 17 |
| Empiric antibiotic choices in children with CAP should be based on the patient’s age and severity of illness, and local resistance patterns of pathogens. | C | 7, 25, 26 |
| Oral amoxicillin and intravenous penicillin G are equally effective in the treatment of hospitalized children with nonsevere CAP. However, amoxicillin is generally more cost-effective. | B | 27 |
| Macrolides are the empiric antibiotics of choice for children five to 16 years of age with CAP because of their activity against Mycoplasma pneumoniae and Chlamydophila pneumoniae. | C | 7, 25, 26 |
| Routine childhood immunization with the pneumococcal conjugate vaccine significantly reduces the incidence of invasive pneumococcal disease in children. | A | 8, 36, 37 |
Etiology
Viruses cause a significant percentage of CAP infections, especially in children younger than two years (Table 1).3,4 The prevalence of viral pneumonia decreases with age.3 Respiratory syncytial virus, influenza A, and parainfluenza types 1 through 3 are the most common viral agents. Other viral pathogens include adenovirus, rhinovirus, influenza B, and enteroviruses.5 Human metapneumovirus has been identified as a common cause of CAP in cases previously classified as virus-negative. The spectrum of illness caused by metapneumovirus is similar to that of respiratory syncytial virus.6 Mixed viral and bacterial infection accounts for 30 to 50 percent of CAP infections in children.7
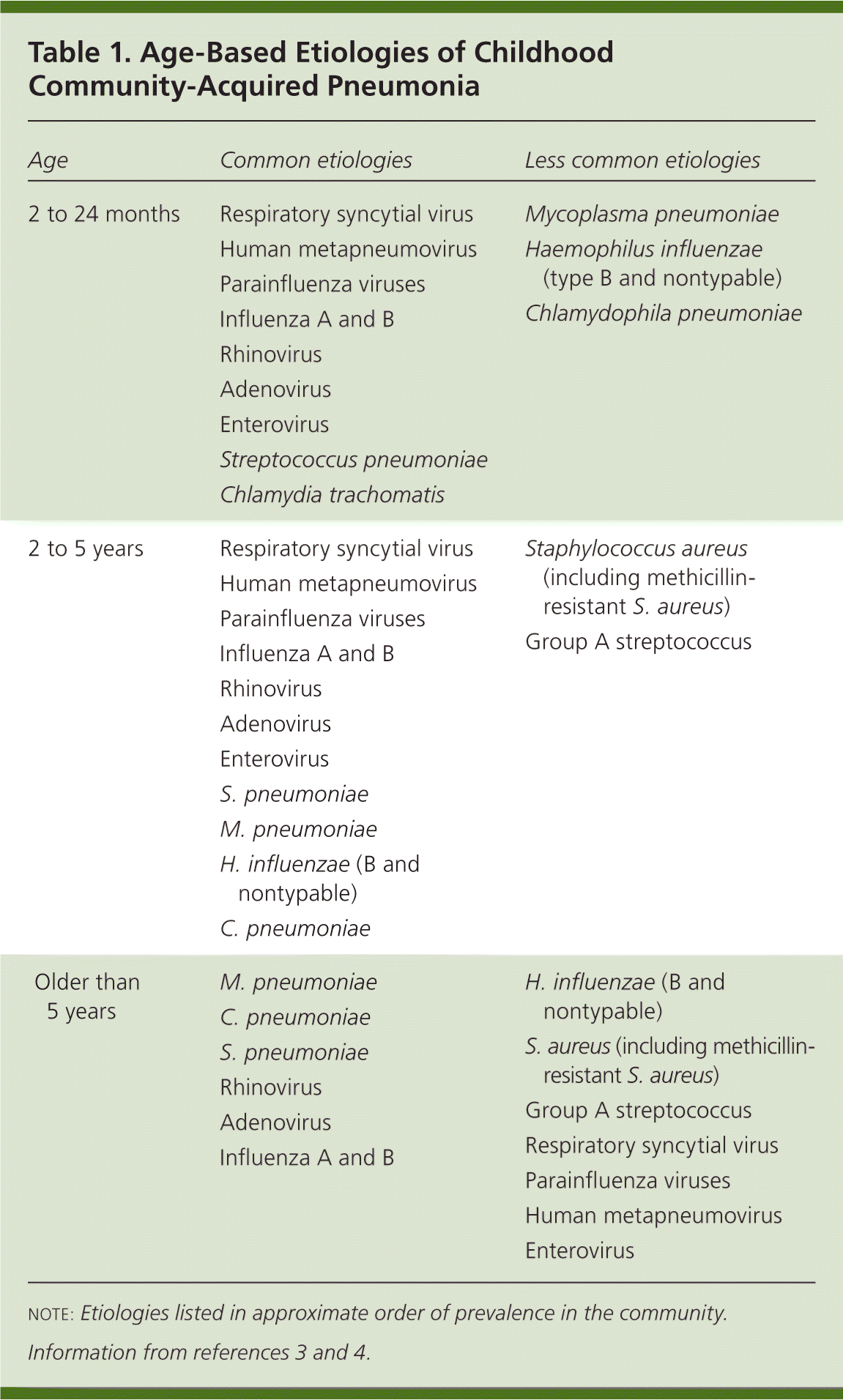
| Age | Common etiologies | Less common etiologies |
|---|---|---|
| 2 to 24 months |
|
|
| 2 to 5 years |
|
|
| Older than 5 years |
|
|
Streptococcus pneumoniae is the most common bacterial cause of CAP. The widespread use of pneumococcal immunization has reduced the incidence of invasive disease.8 Children with underlying conditions and those who attend child care are at higher risk of invasive pneumococcal disease. Breast-feeding seems to be protective. Penicillin-resistant S. pneumoniae infections can occur in children with recent antibiotic use.9
Mycoplasma pneumoniae, Chlamydophila pneumoniae, and S. pneumoniae are the predominant etiologies of CAP in school-aged children.3 Haemophilus influenzae and group A streptococcus are less common causes. Staphylococcus aureus, especially methicillin-resistant S. aureus (MRSA), is increasingly common and causes significant morbidity and mortality.10 Identification of S. pneumoniae and S. aureus as pathogens can be problematic because they can be carried asymptomatically.4
STAPHYLOCOCCUS AUREUS
S. aureus accounts for 3 to 5 percent of CAP infections and is a complication of seasonal and pandemic influenza in children and young adults.11 Reports of S. aureus infection associated with influenza-related deaths in children have raised concerns that this syndrome is increasing in frequency.10,12 In the past decade, case series have estimated that 41 to 88 percent of patients with S. aureus pneumonia have MRSA isolates.10,13
The potential severity of community-acquired staphylococcal pneumonia in children is illustrated by a study of admissions to three children’s hospitals during the autumn and winter of 2006 and 2007.10 Of 30 patients, 25 (83 percent) required intensive care unit treatment, 21 (70 percent) required mechanical ventilation, five (17 percent) required extracorporeal membrane oxygenation, and five (17 percent) died.
The diagnosis of staphylococcal pneumonia is challenging. No specific symptoms, clinical signs, or imaging or laboratory findings have been identified as having high specificity for staphylococcal pneumonia. Cavitation on chest imaging, a possible marker for staphylococcal pneumonia in adults,14 was identified in only two of 30 children hospitalized for CAP.10 Physicians should have a high index of suspicion for S. aureus infection in children with CAP, especially those who are severely ill, have current or recent influenza, or whose symptoms do not improve with beta-lactam or macrolide antibiotic therapy.
Diagnosis
First impressions are important in the clinical diagnosis of CAP in children. Common physical findings include fever, tachypnea, increasingly labored breathing, rhonchi, crackles, and wheezing. Hydration status, activity level, and oxygen saturation are important and may indicate the need for hospitalization.7
Tachypnea seems to be the most significant clinical sign. To be measured accurately, the respiratory rate must be counted over a full minute when the child is quiet.15 In febrile children, the absence of tachypnea has a high negative predictive value (97.4 percent) for pneumonia.1,15,16 Conversely, the presence of tachypnea in febrile children has a low positive predictive value (20.1 percent).15 Fever alone can increase the respiratory rate by 10 breaths per minute per degree Celsius.16 In febrile children with tachypnea, findings of chest retractions, grunting, nasal flaring, and crepitation increase the likelihood of pneumonia.16 The World Health Organization uses tachypnea in the presence of cough as the diagnostic criterion of pneumonia in developing countries where chest radiography is not readily available17,18 (Table 218 ). [ corrected]
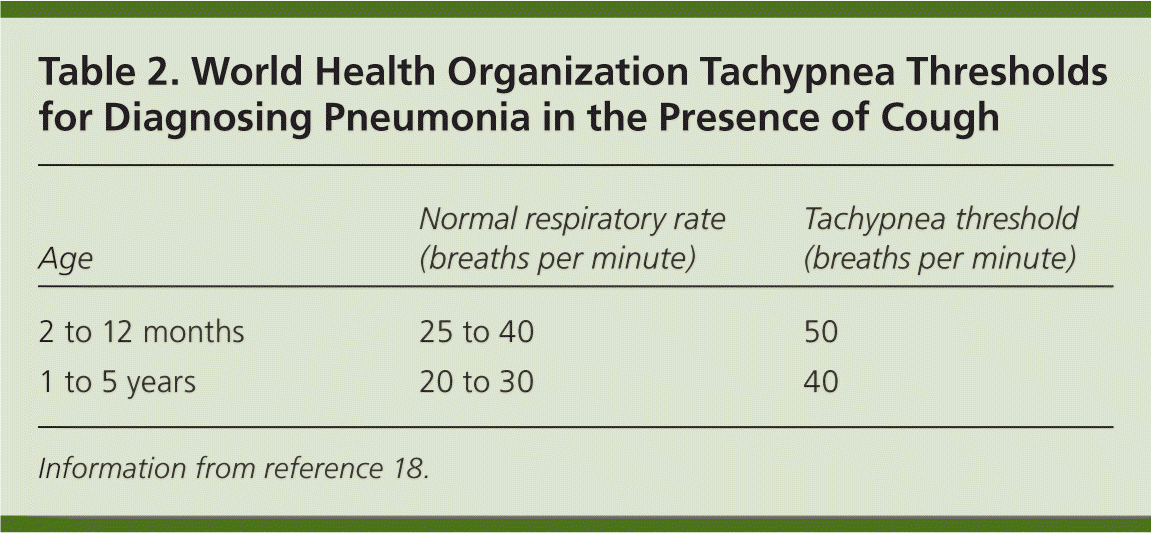
| Age | Normal respiratory rate (breaths per minute) | Tachypnea threshold (breaths per minute) |
|---|---|---|
| 2 to 12 months | 25 to 40 | 50 |
| 1 to 5 years | 20 to 30 | 40 |
Chest radiography is often used to diagnose CAP. Many studies use chest radiography as the preferred diagnostic modality, but positive findings have not been shown to improve clinical outcomes or significantly change treatment.17 Chest imaging is most useful when the diagnosis is uncertain or when the findings from the history and physical examination are inconsistent. Antigenic tests are available to aid in the detection of respiratory syncytial virus and influenza A and B. The Infectious Diseases Society of America recommends that all persons with fever and respiratory symptoms be tested for influenza when it is present in the community.19 The American Academy of Pediatrics recommends testing for respiratory syncytial virus only when the diagnosis is unclear.20
Bacterial pneumonia may be suspected based on radiographic findings; however, these findings are not highly specific. Pleural effusion on chest radiography is the most significant predictor of bacterial pneumonia.5 Alveolar infiltrate is more suggestive of bacterial than viral infection, especially if the infiltrate is lobar.21 Interstitial infiltrates can occur in viral or bacterial infections.21 Positive radiographic findings may be absent in patients with early bacterial pneumonia.16
C-reactive protein and procalcitonin levels, white blood cell count, and erythrocyte sedimentation rate have limited use in the diagnosis of bacterial pneumonia.22 One older study of children younger than 16 years showed that 93 percent of those with a white blood cell count greater than 20,000 cells per mm3 (20 × 109 per L) improved with antibiotic therapy, compared with only 50 percent of those with a count of less than 10,000 cells per mm3 (10 × 109 per L).23 The improvement with antibiotics was significant in patients with a white blood cell count greater than 15,000 cells per mm3 (15 × 109 per L), which suggests an association with bacterial pneumonia. Sputum cultures are difficult to obtain and are of limited use in diagnosis or therapy. Blood culture results have not been shown to change clinical management and often do not yield a pathogen.24
Antibiotic Therapy
The initial antibiotic treatment of CAP is empiric because the pathogen is rarely known at the time of diagnosis (Tables 3 and 4).7,25,26 Empiric antibiotic choices should be based on the patient’s age and severity of illness, and local resistance patterns of common pathogens.7,25,26 Few large randomized controlled trials have compared antibiotics in the treatment of childhood CAP, but several organizations have published treatment guidelines.7,25,26 Oral administration of antibiotics is preferred except when the patient cannot tolerate oral therapy or has severe CAP.26

| Age | Alberta guideline25 | Cincinnati guideline7 | |
|---|---|---|---|
| 60 days to 5 years* | Preferred regimens | ||
| Amoxicillin† | Amoxicillin | ||
| 40 mg per kg per day for 7 to 10 days | 80 to 90 mg per kg per day, in two divided doses, for 7 to 10 days | ||
| or | |||
| 90 mg per kg per day, in three divided doses, for 7 to 10 days | |||
| Alternative regimens for patients allergic to penicillin or beta-lactam antibiotics | |||
| Azithromycin (Zithromax) | Azithromycin | ||
| Day 1: 10 mg per kg | Day 1: 10 mg per kg | ||
| Days 2 through 5: 5 mg per kg per day | Days 2 through 5: 5 mg per kg per day | ||
| Clarithromycin (Biaxin) | Clarithromycin | ||
| 15 mg per kg per day, in two divided doses, for 7 to 10 days | 15 mg per kg per day, in two divided doses, for 7 to 10 days | ||
| Erythromycin | Cefprozil (Cefzil) | ||
| 40 mg per kg per day, in four divided doses, for 7 to 10 days | 30 mg per kg per day, in two divided doses, for 7 to 10 days | ||
| Cefuroxime (Ceftin) | |||
| 30 mg per kg per day, in two divided doses, for 7 to 10 days | |||
| Ceftriaxone (Rocephin)‡ | |||
| Single dose of 50 mg per kg, administered intramuscularly | |||
| 5 to 16 years | Azithromycin§ | Azithromycin∥ | |
| Day 1: 10 mg per kg | Day 1: 10 mg per kg | ||
| Days 2 through 5: 5 mg per kg per day | Days 2 through 5: 5 mg per kg per day | ||

| Age | Alberta guideline25 | Cincinnati guideline7 | |
|---|---|---|---|
| 60 days to 5 years* | Cefuroxime (Zinacef) | See Table 3 | |
| 150 mg per kg per day IV, in divided doses, given every 8 hours for 10 to 14 days | |||
| In critically ill patients: | Not applicable | ||
| Cefuroxime | |||
| 150 mg per kg per day IV, in divided doses, given every 8 hours for 10 to 14 days | |||
| plus | |||
| Erythromycin | |||
| 40 mg per kg per day IV or orally, in divided doses, given every 6 hours for 10 to 14 days | |||
| or | |||
| Cefotaxime (Claforan) | |||
| 200 mg per kg per day IV, in divided doses, given every 8 hours for 10 to 14 days | |||
| plus | |||
| Cloxacillin (no longer available in the United States) | |||
| 150 to 200 mg per kg per day IV, in divided doses, given every 6 hours for 10 to 14 days | |||
| 5 to 16 years | Cefuroxime | See Table 3 | |
| 150 mg per kg per day IV, in divided doses, given every 8 hours for 10 to 14 days | |||
| plus | |||
| Erythromycin | |||
| 40 mg per kg per day IV or orally, in divided doses, given every 6 hours for 10 to 14 days | |||
| or | |||
| Azithromycin (Zithromax) | |||
| Day 1: 10 mg per kg IV or orally | |||
| Days 2 through 5: 5 to 10 mg per kg per day IV or orally | |||
A 2007 study showed that oral amoxicillin and intravenous penicillin G were equally effective in the treatment of hospitalized children with nonsevere CAP.27 A subsequent study showed that oral amoxicillin was more cost-effective for most hospitalized children with CAP.28 Patients receiving parenteral therapy may be switched to oral treatment once they are afebrile and improving clinically, can tolerate oral intake, and have no complications.25 Amoxicillin is the drug of choice for patients 60 days to five years of age because of its activity against S. pneumoniae. Macrolides or cephalosporins can be used in patients with penicillin allergy. Macrolides are the treatment of choice for children five to 16 years of age because of their activity against M. pneumoniae and C. pneumoniae.
For patients with more severe disease, amoxicillin (or another beta-lactam antibiotic) may be combined with a macrolide.5 If MRSA infection is suspected, empiric therapy with vancomycin should be started (15 mg per kg intravenously every six hours). Clindamycin (10 to 13 mg per kg orally or intravenously every six to eight hours) may be used if the patient is stable without bacteremia, and if the local resistance rate to clindamycin is less than 10 percent. Linezolid (Zyvox) is another alternative (10 mg per kg orally or intravenously every eight hours in children younger than 12 years, or 600 mg orally or intravenously twice per day in children 12 years and older).29
DURATION OF THERAPY
No randomized controlled trials have established the optimal duration of therapy for children with uncomplicated CAP.26 In most cases, seven to 10 days of empiric outpatient therapy is sufficient.7,25,26 Azithromycin (Zithromax) should be continued for five days. Patients should be reevaluated 24 to 48 hours after the initiation of empiric therapy.7 Ineffective empiric therapy may be the result of inappropriate drug selection, resistance to the initial agents, or development of complications.7 Some evidence suggests that short-course, high-dose amoxicillin therapy (90 mg per kg per day for five days) may reduce the risk of carriage of penicillin-nonsusceptible pneumococci compared with longer-course, lower-dose regimens (40 mg per kg per day for 10 days).30
Supportive Care
Children with pneumonia are usually febrile. They may have localized chest pain, referred pain to the abdomen, headache, or arthralgia. Pleural pain and abdominal pain may interfere with effective cough. These symptoms may be controlled with weight-appropriate doses of antipyretics and analgesics, such as acetaminophen or ibuprofen. Aspirin is not recommended for children because of the risk of Reye syndrome.26
Inpatient Care
Pneumonia accounted for 161,000 (7.8 percent) of the childhood hospitalizations in 2000 (excluding neonates and pregnant adolescents).31 The average length of hospitalization for pneumonia in children is approximately three days.32 The decision to admit a child or adolescent with pneumonia should be made on an individual basis and should be based on multiple clinical and social factors. Most infants younger than four months should be admitted initially unless a viral etiology or Chlamydia trachomatis infection is suspected, or unless the infant is relatively asymptomatic and close follow-up can be ensured.26 Indications for hospital admission are shown in Table 5.7,26
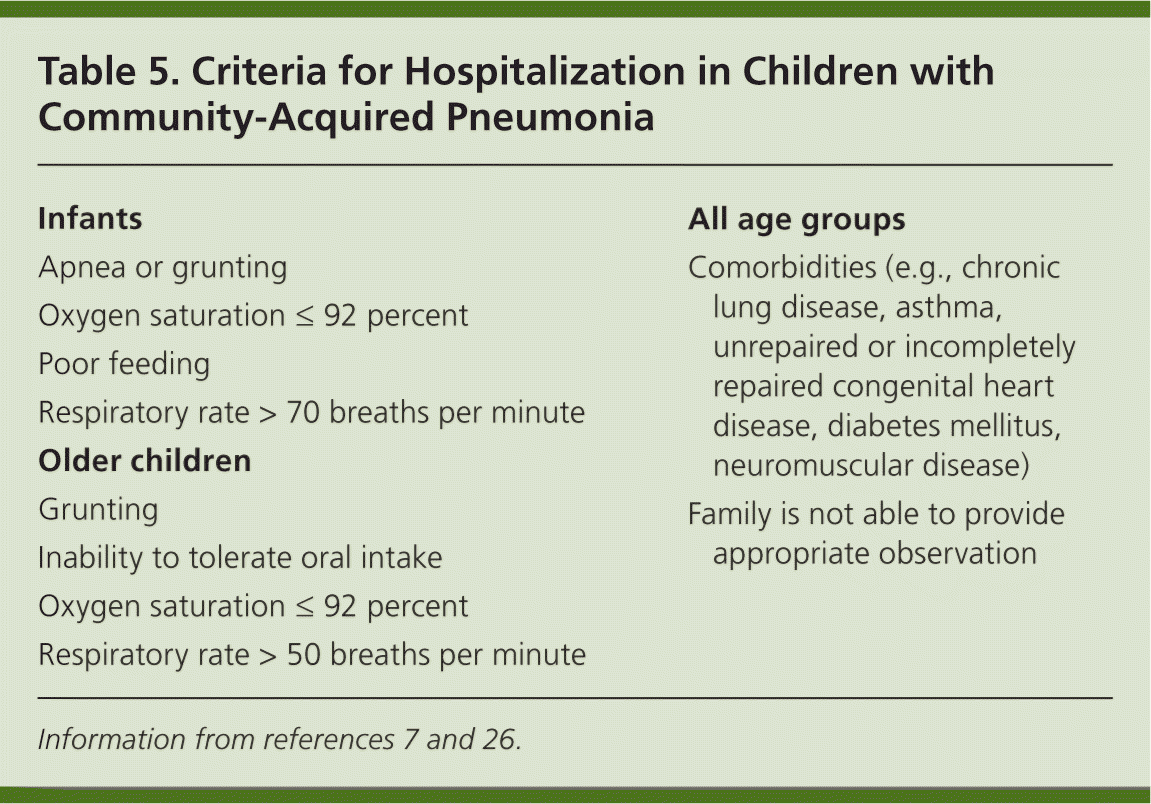
| Infants |
| Apnea or grunting |
| Oxygen saturation ≤ 92 percent |
| Poor feeding |
| Respiratory rate > 70 breaths per minute |
| Older children |
| Grunting |
| Inability to tolerate oral intake |
| Oxygen saturation ≤ 92 percent |
| Respiratory rate > 50 breaths per minute |
| All age groups |
| Comorbidities (e.g., chronic lung disease, asthma, unrepaired or incompletely repaired congenital heart disease, diabetes mellitus, neuromuscular disease) |
| Family is not able to provide appropriate observation |
Clinical parameters that should be monitored include temperature, respiratory rate, heart rate, oxygen saturation, work of breathing (presence of retractions, nasal flaring, grunting), and auscultatory findings.26 Hypoxic infants and children may not appear cyanotic, and agitation may be the only indication of hypoxia.26 Patients whose oxygen saturation is less than 92 percent should be treated with supplemental oxygen via nasal cannula, face mask, or (in infants) an oxygen hood.26 No strong evidence suggests that any of these methods is more effective than another.33 Patients who receive supplemental oxygen should have oxygen saturation levels evaluated every four hours.26 Children in severe respiratory distress should be evaluated for hypercapnia by arterial blood gas measurements because oxygenation may be preserved.26 Children who are vomiting, have suboptimal oral intake, or are severely ill require enteral or parenteral fluid therapy. Chest physiotherapy has no effect on length of hospital stay, duration of fever, or chest radiologic findings in patients with pneumonia.34
Mortality from childhood pneumonia in developed countries is low, but patients may require intensive care. Transfer to a pediatric intensive care unit is warranted when the child cannot maintain an oxygen saturation level greater than 92 percent despite a fraction of inspired oxygen greater than 0.6, the patient is in shock, the respiratory rate and pulse rate are rising, and the child shows evidence of severe respiratory distress and exhaustion (with or without a rise in partial arterial carbon dioxide tension), or when the child has recurrent episodes of apnea or slow, irregular breathing.26
Prevention
Current treatment guidelines suggest several interventions to prevent CAP. These include frequent handwashing, avoiding tobacco smoke, promoting breastfeeding, reducing exposure to other children, and immunization.7,25 The pneumococcal conjugate vaccine (Prevnar 13) is approved for the prevention of invasive pneumococcal disease in children six weeks to 71 months of age.8,35–37 Children should also be vaccinated against other potential causes of pneumonia, including influenza, H. influenzae type b, pertussis, varicella, and measles.
Data Sources: PubMed Clinical Queries was searched using the key terms community acquired pneumonia, pediatrics, diagnosis, treatment, pneumococcal pneumonia, pneumococcal vaccine, and MRSA pneumonia. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. Also searched were the Agency for Healthcare Research and Quality and the Cochrane databases. Essential Evidence Plus, which included the Cincinnati guideline, was also reviewed. References from selected articles and in UpToDate were searched. Search dates: October and November 2010.