
This is a corrected version of the article that appeared in print.
Am Fam Physician. 2014;89(4):273-278
Author disclosure: No relevant financial affiliations.
Thyroid disease is the second most common endocrine disorder affecting women of reproductive age, and when untreated during pregnancy is associated with an increased risk of miscarriage, placental abruption, hypertensive disorders, and growth restriction. Current guidelines recommend targeted screening of women at high risk, including those with a history of thyroid disease, type 1 diabetes mellitus, or other autoimmune disease; current or past use of thyroid therapy; or a family history of autoimmune thyroid disease. Appropriate management results in improved outcomes, demonstrating the importance of proper diagnosis and treatment. In women with hypothyroidism, levothyroxine is titrated to achieve a goal serum thyroid-stimulating hormone level less than 2.5 mIU per L. The preferred treatment for hyperthyroidism is antithyroid medications, with a goal of maintaining a serum free thyroxine level in the upper one-third of the normal range. Postpartum thyroiditis is the most common form of postpartum thyroid dysfunction and may present as hyper- or hypothyroidism. Symptomatic treatment is recommended for the former; levothyroxine is indicated for the latter in women who are symptomatic, breastfeeding, or who wish to become pregnant.
Thyroid disease is second only to diabetes mellitus as the most common endocrinopathy that occurs in women during their reproductive years. Symptoms of thyroid disease often mimic common symptoms of pregnancy, making it challenging to identify. Poorly controlled thyroid disease is associated with adverse outcomes during pregnancy, and treatment is an essential part of prenatal care to ensure maternal and fetal well-being.1–3
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| The optimal method to assess serum FT4 during pregnancy uses direct measurement techniques. Serum TSH is a more accurate indicator of maternal thyroid status than alternative FT4 assay methods. | C | 3, 6 |
| Targeted screening for thyroid disease should be performed in pregnant women at high risk, including those with a history of thyroid disease, type 1 diabetes mellitus, or other autoimmune disease; current or past use of thyroid therapy; or a family history of autoimmune thyroid disease. | C | 2, 3 |
| Hypothyroidism during pregnancy should be treated with levothyroxine, with a serum TSH goal of less than 2.5 mIU per L. | A | 1–3 |
| Serum TSH should be measured in pregnant women who are being treated for hypothyroidism at four to six weeks' gestation, then every four to six weeks until 20 weeks' gestation and on a stable medication dosage, then again at 24 to 28 weeks' and 32 to 34 weeks' gestation. | C | 2, 3 |
| Propylthiouracil is the preferred agent for the treatment of hyperthyroidism during the first trimester of pregnancy and in women with methimazole (Tapazole) allergy and hyperthyroidism. Consideration should be given to switching to methimazole after the first trimester, and the dosage should be adjusted to maintain a serum FT4 level in the upper one-third of the normal range. | C | 3 |
| In pregnant women who are being treated for hyperthyroidism, serum TSH and FT4 should be measured every two weeks until the patient is on a stable medication dosage. | C | 2, 3 |
Thyroid Function Tests in Pregnancy
To understand abnormal thyroid function in pregnancy, a review of normal physiologic changes is warranted (Table 1).4 Because of the estrogen-mediated increase in thyroid-binding globulin, the increased volume of distribution of thyroid hormone, and the placental metabolism and transport of maternal thyroxine, there is a 20% to 40% increase in the thyroid hormone requirement as early as the fourth week of gestation.5

| Maternal condition | Thyroid-stimulating hormone | Free thyroxine | Free thyroxine index | Total thyroxine | Triiodothyronine | Resin triiodothyronine uptake |
|---|---|---|---|---|---|---|
| Hyperthyroidism | Decrease | Increase | Increase | Increase | Increase or no change | Increase |
| Hypothyroidism | Increase | Decrease | Decrease | Decrease | Decrease or no change | Decrease |
| Normal pregnancy | Decrease | No change | No change | Increase | Increase | Decrease |
During pregnancy, reference ranges for thyroid-stimulating hormone (TSH) are lower because of the cross-reactivity of the alpha subunit of human chorionic gonadotropin with the TSH receptor.2,3 Changes in serum-binding protein levels can influence measurements of free thyroxine (FT4) that rely on estimates rather than direct measurements, resulting in inaccurate reported values.6 Physicians should know the limitations of locally available assay methods. When preferred FT4 assay techniques are unavailable, a serum TSH level is a more accurate assessment of maternal thyroid status, and measurements of total thyroxine and the FT4 index can be used instead.3,6 Trimester-specific ranges for common serum thyroid studies are shown in Table 2.3,7 [ corrected]

| Test | Nonpregnant | First trimester | Second trimester | Third trimester |
|---|---|---|---|---|
| Thyroid-stimulating hormone (mIU per L) | 0.3 to 4.3 | 0.1 to 2.5 | 0.2 to 3.0 | 0.3 to 3.0 |
| Thyroxine-binding globulin (mg per dL) | 1.3 to 3.0 | 1.8 to 3.2 | 2.8 to 4.0 | 2.6 to 4.2 |
| Thyroxine, free (ng per dL) | 0.8 to 1.7 | 0.8 to 1.2 | 0.6 to 1.0 | 0.5 to 0.8 |
| Thyroxine, total (mcg per dL) | 5.4 to 11.7 | 6.5 to 10.1 | 7.5 to 10.3 | 6.3 to 9.7 |
| Triiodothyronine, free (pg per mL) | 2.4 to 4.2 | 4.1 to 4.4 | 4.0 to 4.2 | Not reported |
| Triiodothyronine, total (ng per dL) | 77 to 135 | 97 to 149 | 117 to 169 | 123 to 162 |
Screening
The Endocrine Society recommends screening only pregnant women at high risk of thyroid disease using serum TSH measurement (Table 3).2,3 Although one study found that selectively screening women at high risk would miss 30% of those with overt or subclinical hypothyroidism,8 a randomized controlled trial of 4,562 women did not show a reduction in adverse outcomes in those who were universally screened vs. case finding.9

| Current thyroid therapy | |
| Family history of autoimmune thyroid disease | |
| Goiter | |
| History of: | |
| Autoimmune disorder | |
| High-dose neck radiation | |
| Postpartum thyroid dysfunction | |
| Previous delivery of infant with thyroid disease | |
| Therapy for hyperthyroidism | |
| Type 1 diabetes mellitus | |
Preconception Counseling
Women with hypothyroidism should be counseled about the importance of achieving euthyroidism before conception because of the risk of decreased fertility and miscarriage.1–3 They must also understand the importance of immediate monitoring at the onset of pregnancy, because by the first prenatal visit, many of these patients will already have an elevated TSH level consistent with uncontrolled hypothyroidism.5 Euthyroid women who are taking a stable dosage of levothyroxine should be counseled to notify their physician and independently increase their medication by two additional doses per week after a missed menstrual cycle or positive home pregnancy test.3 In a study of 48 women treated for hypothyroidism with a normal prepregnancy serum TSH level, increasing levothyroxine by two doses per week prevented maternal TSH elevation above 2.5 mIU per L and above 5 mIU per L in 85% and 100% of patients, respectively, with only two patients requiring a subsequent dose reduction.5
Preconception counseling for women with known hyperthyroidism should include discussion of available treatments and potential adverse effects, as well as the impact on future pregnancies. Standard treatments include long-term antithyroid medication, radioactive iodine ablation, and near-total thyroidectomy. Potential adverse fetal effects of antithyroid medications include congenital abnormalities and neonatal hypothyroidism caused by transplacental transfer.2,3 Although radioactive iodine ablation is not associated with long-term consequences on gonadal function, fertility, or pregnancy outcomes, it is customary to wait six months after the therapeutic dose is administered before attempting conception.8 Radioactive ablation and surgery can increase the risk of neonatal goiter and hyperthyroidism because of the absence of maternal antithyroid medication, which crosses the placenta and counteracts the stimulatory effect of thyrotropin receptor antibodies on the fetal thyroid.2,3 The importance of achieving and maintaining euthyroidism before conception should be emphasized, because a significant increase in congenital malformations has been reported when hyperthyroidism is not controlled in the first trimester of pregnancy.10
Hypothyroidism
The incidence of hypothyroidism during pregnancy is estimated to be 0.3% to 0.5% for overt hypothyroidism and 2% to 3% for subclinical hypothyroidism.11 Overt hypothyroidism is defined as thyroid hormone deficiency with low FT4 and elevated TSH levels, whereas subclinical hypothyroidism refers to asymptomatic individuals with elevated TSH and normal FT4 levels.
Worldwide, the most common cause of hypothyroidism is iodine deficiency. In iodine-sufficient regions, the most common causes are autoimmune thyroiditis and iatrogenic hypothyroidism after treatment for hyperthyroidism.11 Symptoms such as fatigue, weight gain, decreased exercise capacity, and constipation are often confused with common symptoms of pregnancy; other symptoms such as hair loss, dry skin, and bradycardia may be evident only in more symptomatic persons.
Overt and subclinical hypothyroidism have been associated with adverse effects on pregnancy and fetal development (Table 4).1–3 These maternal conditions contribute to an increased risk of adverse neonatal outcomes, including preterm birth, low birth weight, and increased perinatal morbidity and mortality.12 Childhood neurodevelopment also seems to be contingent on thyroid hormone regulation; impairment of neuropsychologic developmental indices and school learning abilities has been noted in children whose mothers had poorly controlled hypothyroidism during pregnancy.2,3,13

| Condition | Preconception | Pregnancy | Postpartum | Medications |
|---|---|---|---|---|
| Hyperthyroidism, overt | Congenital malformations |
| — |
|
| Hyperthyroidism, subclinical | — |
| — |
|
| Hypothyroidism, overt | Decreased fertility, increased miscarriage |
| Maternal thyroid dysfunction, hemorrhage |
|
| Hypothyroidism, subclinical | Effects similar to overt hypothyroidism, but less documentation exists | |||
Levothyroxine is the mainstay of treatment for maternal hypothyroidism (Table 5).2,3,14–16 The increment of dose adjustment generally is based on the degree of TSH elevation (Table 6).17 Serum TSH should be measured every four to six weeks until 20 weeks' gestation and until the patient is on a stable medication dose; it should be measured again at 24 to 28 weeks' and 32 to 34 weeks' gestation.2,3,17 Antenatal testing is not recommended in women with well-controlled hypothyroidism, but it should be considered in patients with coexisting maternal or obstetric indications. After delivery, levothyroxine should be decreased to the prepregnancy dosage over a four-week period, and further adjustment should be guided by TSH levels four to six weeks after delivery.2
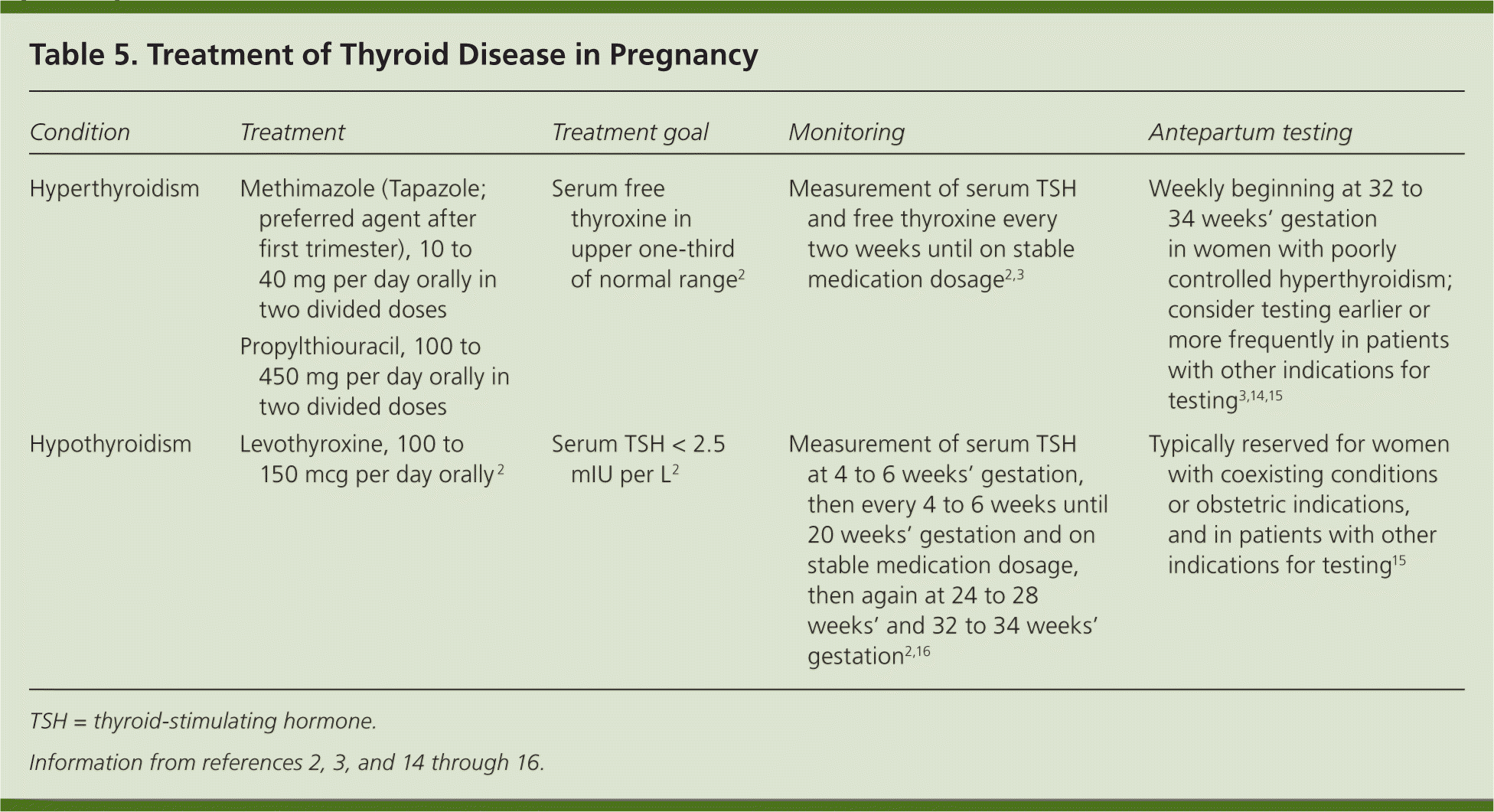
| Condition | Treatment | Treatment goal | Monitoring | Antepartum testing |
|---|---|---|---|---|
| Hyperthyroidism | Methimazole (Tapazole; preferred agent after first trimester), 10 to 40 mg per day orally in two divided doses | Serum free thyroxine in upper one-third of normal range2 | Measurement of serum TSH and free thyroxine every two weeks until on stable medication dosage2,3 | Weekly beginning at 32 to 34 weeks' gestation in women with poorly controlled hyperthyroidism; consider testing earlier or more frequently in patients with other indications for testing3,14,15 |
| Propylthiouracil, 100 to 450 mg per day orally in two divided doses | ||||
| Hypothyroidism | Levothyroxine, 100 to 150 mcg per day orally 2 | Serum TSH < 2.5 mIU per L2 | Measurement of serum TSH at 4 to 6 weeks' gestation, then every 4 to 6 weeks until 20 weeks' gestation and on stable medication dosage, then again at 24 to 28 weeks' and 32 to 34 weeks' gestation2,16 | Typically reserved for women with coexisting conditions or obstetric indications, and in patients with other indications for testing15 |

| Thyroid-stimulating hormone level (mIU per L) | Levothyroxine dosage increase (mcg per day) |
|---|---|
| 5 to < 10 | 25 to 50 |
| 10 to 20 | 50 to 75 |
| > 20 | 75 to 100 |
Treatment seems to reduce the incidence of miscarriage and preterm birth, and to improve fetal intellectual development; however, it has little impact on hypertensive disorders and placental abruption.1
Hyperthyroidism
Hyperthyroidism is less common than hypothyroidism, with an approximate incidence during pregnancy of 0.2%.11 Overt hyperthyroidism is defined as elevated FT4 and low TSH levels, whereas subclinical hyperthyroidism is defined as asymptomatic low TSH and normal FT4 levels. Clinical symptoms of hyperthyroidism include tachycardia, nervousness, tremor, sweating, heat intolerance, proximal muscle weakness, frequent bowel movements, decreased exercise tolerance, and hypertension.
Graves disease, which accounts for 95% of cases of hyperthyroidism, is an autoimmune disorder mediated by stimulatory antibodies against the TSH receptor. Other less common causes of hyperthyroidism include gestational trophoblastic disease, nodular goiter or solitary toxic adenoma, viral thyroiditis, and tumors of the pituitary gland or ovary. Transient hyperthyroidism may also be associated with hyperemesis gravidarum and gestational transient thyrotoxicity, most likely resulting from the stimulatory effect of human chorionic gonadotropin on the thyroid.11 Although the radioactive iodine uptake scan used in the diagnosis of hyperthyroidism is contra-indicated during pregnancy, testing for the presence of antithyroid antibodies can be diagnostically useful.
The natural history of hyperthyroid disorders varies with the underlying etiology. Graves disease is typically characterized by an initial exacerbation of symptoms in the first trimester, and is thought to be caused by the initial stimulatory effect of human chorionic gonadotropin on the thyroid. Symptoms usually improve during the second half of the pregnancy, only to worsen again in the postpartum period.11 Overt hyperthyroidism that is inadequately treated is associated with an increased risk of adverse maternal and neonatal outcomes (Table 4).1–3 However, one large prospective study of more than 25,000 pregnant women with subclinical hyperthyroidism showed no increase in adverse pregnancy outcomes; therefore, treatment is not recommended in these cases.18
Overt hyperthyroidism during pregnancy is treated with methimazole (Tapazole) or propylthiouracil (Table 5).2,3,14–16 Because the use of methimazole is associated with birth defects, including aplasia cutis and choanal or esophageal atresia,16,19 propylthiouracil is the preferred medication during the first trimester.3 However, it is recommended that physicians consider switching to methimazole after the first trimester because the risk of liver failure associated with propylthiouracil use is greater than the risk of congenital abnormalities.2,3
The main concern in women with hyperthyroidism is the potential effect on the fetus. Thyroid receptor antibodies should be measured by the end of the second trimester in women with active Graves disease, a history of Graves disease treated with radioactive iodine or thyroidectomy, or a history of a previous infant with Graves disease.2,3,20 In women at high risk, including those receiving antithyroid medication and those with poorly controlled hyperthyroidism or high thyrotoxin receptor antibody levels, fetal ultrasonography should be performed monthly after 20 weeks' gestation to detect evidence of fetal thyroid dysfunction (e.g., growth restriction, hydrops, goiter, cardiac failure).2,3,21 These women should also undergo antepartum testing at least weekly beginning at 32 to 34 weeks' gestation (or earlier in particularly high-risk situations).22
Postpartum Thyroid Dysfunction
The most common cause of postpartum thyroid dysfunction is postpartum thyroiditis, which affects 1.1% to 21.1% of women.23 Postpartum thyroiditis is defined as an abnormal TSH level within the first 12 months postpartum in the absence of a toxic thyroid nodule or thyrotoxin receptor antibodies.23 Clinical symptoms can mimic the typical fatigue following delivery, as well as postpartum depression and Graves disease; a thorough assessment is required to differentiate these conditions. A radioactive iodine uptake scan can help distinguish postpartum thyroiditis from Graves disease, but is contraindicated in breastfeeding women. Patients must limit close contact with others for a time after the study.
The clinical course of postpartum thyroiditis varies: approximately 25% of patients present with symptoms of hyperthyroidism, followed by hypothyroidism and then recovery; 43% present with symptoms of hypothyroidism; and 32% present with hyperthyroidism.3 Because the hyperthyroid phase of postpartum thyroiditis is caused by autoimmune destruction of the thyroid, resulting in release of stored thyroid hormone, antithyroid medications are not typically beneficial and treatment is generally symptomatic, using peripheral beta antagonists. Differentiation of the hyperthyroid phase of postpartum thyroiditis from Graves disease is important because Graves disease requires antithyroid therapy. In contrast, postpartum hypothyroidism should be treated with levothyroxine in women who are symptomatic or breastfeeding, or who wish to become pregnant, and may require lifetime supplementation.3,5
Women with a history of type 1 diabetes and women with thyroglobulin or thyroperoxidase autoantibodies are at increased risk of postpartum thyroiditis.14,15 Asymptomatic women should be screened at three and six months postpartum using serum TSH measurement.2 Additionally, women with a history of postpartum thyroiditis are at increased risk of permanent hypothyroidism and should be screened annually thereafter.2,3,24
Data Sources: Essential Evidence Plus was searched using PubMed, and OVID was searched. Key words were thyroid disease and pregnancy. Article selection was limited to human studies, original research, systematic reviews, and current clinical practice guidelines. Search date: August 22, 2013.
