
Am Fam Physician. 2016;93(7):560-569
Patient information: See related handout on recurrent urinary tract infections, written by the authors of this article.
Author disclosure: No relevant financial affiliations.
Recurrent urinary tract infections (UTIs) are common in women, including healthy women with normal genitourinary anatomy. Recurrent UTI is typically defined as three or more UTIs within 12 months, or two or more occurrences within six months. The same species that caused previous infections is typically responsible for recurrences. In premenopausal women, sexual intercourse three or more times per week, spermicide use, new or multiple sex partners, and having a UTI before 15 years of age are established risk factors. In postmenopausal women, risk is primarily increased by sequelae of lower estrogen levels. Episodes of recurrent UTI are typically characterized by dysuria and urinary frequency or hesitancy. Findings from the history or physical examination that suggest complicated infection or another disease process warrant additional evaluation. At least one symptomatic episode should be verified by urine culture to confirm the diagnosis and guide treatment. Imaging is rarely warranted. Short courses of antibiotics are as effective as longer courses. Patient-initiated treatment lowers the cost of diagnosis, number of physician visits, and number of symptomatic days compared with physician-initiated treatment. It also reduces antibiotic exposure compared with antibiotic prophylaxis. Antibiotic prophylaxis effectively limits UTI recurrence but increases the risk of antibiotic resistance and adverse effects. Cranberry products may reduce recurrent UTIs in premenopausal women, but are less effective than antibiotic prophylaxis, and data are conflicting. Optimal dosing is unknown. Postmenopausal women with atrophic vaginitis may benefit from topical estrogen therapy.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| In patients who are candidates for prophylactic or self-initiated treatment of recurrent UTI, at least one episode should be confirmed by a urine culture demonstrating at least 102 bacterial colonies per mL of a known urinary pathogen when the patient is symptomatic. | C | 3, 5, 11, 15, 17, 22 |
| Imaging and cystoscopy are rarely necessary in healthy women with recurrent UTIs, unless risk factors for complicated infection are present. | B | 26–28 |
| A three-day course of trimethoprim/sulfamethoxazole, a one-day course of fosfomycin (Monurol), or a five-day course of nitrofurantoin is as effective as longer treatment courses in achieving clinical cure of an isolated or recurrent UTI. | A | 1, 29–32 |
| Both continuous daily and postcoital low-dose antibiotic prophylaxis regimens decrease recurrence of symptomatic UTIs. | A | 5, 11, 16 |
| Prophylaxis with daily estrogen vaginal cream in postmenopausal women may reduce the risk of future UTIs. | B | 46, 51, 52 |
| Prophylaxis with daily cranberry tablets may reduce the risk of future UTIs in premenopausal women, but data are conflicting. | B | 46–50 |
Recurrent UTI is typically defined as three or more UTIs in 12 months, or two or more infections in six months.2–5 Recurrence is thought to occur by ascent of uropathogens in fecal flora along the urogenital tract and by reemergence of bacteria from intracellular bacterial colonies in uroepithelial cells. In either mechanism, the same species that caused the initial infection is typically the reinfecting agent.5
What Are the Risk Factors for Recurrent UTIs?
Independent risk factors for recurrent UTIs in premenopausal women include sexual intercourse three or more times per week, spermicide use, new or multiple sex partners, and having a UTI before 15 years of age. In postmenopausal women, estrogen deficiency and urinary retention are strong contributors.
EVIDENCE SUMMARY
Frequent intercourse likely causes inoculation of the urethra and bladder by fecal flora, whereas spermicide use disrupts the healthy Lactobacillus flora of the vaginal canal, thereby allowing ascent of uropathogens.6–8 In premenopausal women, intercourse three or more times per week triples the risk of UTI.8 Well-designed case-control studies suggest that body mass index, wiping back-to-front after bowel movements, hot tub use, douching, frequent tampon use, increased hydration, and wearing cotton underwear have no effect on the risk of recurrence.5,6 Postcoital urination seems to have little protective effect but is a reasonable and safe practice.6
In otherwise healthy postmenopausal women, estrogen deficiency is a risk factor for recurrent UTIs because of changes in Lactobacillus flora and vaginal pH.9 Other risk factors in postmenopausal women include incontinence, a postvoid residual urinary volume exceeding 150 mL, structural abnormalities (e.g., cystocele), type 1 or 2 diabetes mellitus, or a history of more than five UTIs.6,9 Activities that increase intra-abdominal pressure (e.g., long-distance walking or traveling) may exacerbate incontinence, cystocele, or postvoid residual urine, and may predispose women who engage in these activities to UTIs.9,10
Is Susceptibility to Recurrent UTIs Inherited?
Inherited factors seem to influence a woman's susceptibility to recurrent UTIs. However, such influences are largely nonmodifiable and therefore do not alter clinical recommendations.
EVIDENCE SUMMARY
Having a first-degree female relative with a history of five or more UTIs is a risk factor for recurrent UTIs.7 Specific inheritance patterns, such as nuanced neutrophil receptors and nonsecretor status of blood-type antigens, may decrease the immune system's ability to clear bacteria or prevent their attachment to uroepithelium. Furthermore, variations in urogenital tract anatomy, including a short urethral-anal distance, may predispose some women to UTIs.5–7
When Is Further Clinical Evaluation Recommended?
A history suggestive of uncomplicated acute cystitis in patients with a previous culture-confirmed UTI is typically sufficient for diagnosis of recurrent infection. Physical examination, laboratory testing, and imaging have limited utility and are not universally recommended.
EVIDENCE SUMMARY
Figure 1 presents a suggested approach to the evaluation and management of recurrent UTIs.3,11–17 Uncomplicated acute cystitis, including recurrent episodes, is typically characterized by a combination of dysuria, urinary frequency, and urinary hesitancy.11,12 Clinicians should be confident in a patient's self-diagnosis of recurrent UTI based on symptoms consistent with previous culture-confirmed infections. In prospective studies, patient suspicion of UTI is more than 85% accurate in predicting culture-positive infections; this is more accurate than urine dipstick testing.13,15,18–22 However, additional evaluation and treatment are warranted in patients with fever, nausea, vomiting, acute back pain, previous urogenital surgery, bladder catheterization, vaginal discharge, pelvic pain, or exposure to a sexually transmitted infection, because these may be signs of a complicated infection or another disease process.13,14,18,23 Pregnancy testing should be considered in premenopausal women. If the patient reports incontinence, overactive bladder, or incomplete bladder emptying, postvoid residual urinary volume and urodynamic testing may be helpful in guiding treatment.14,23
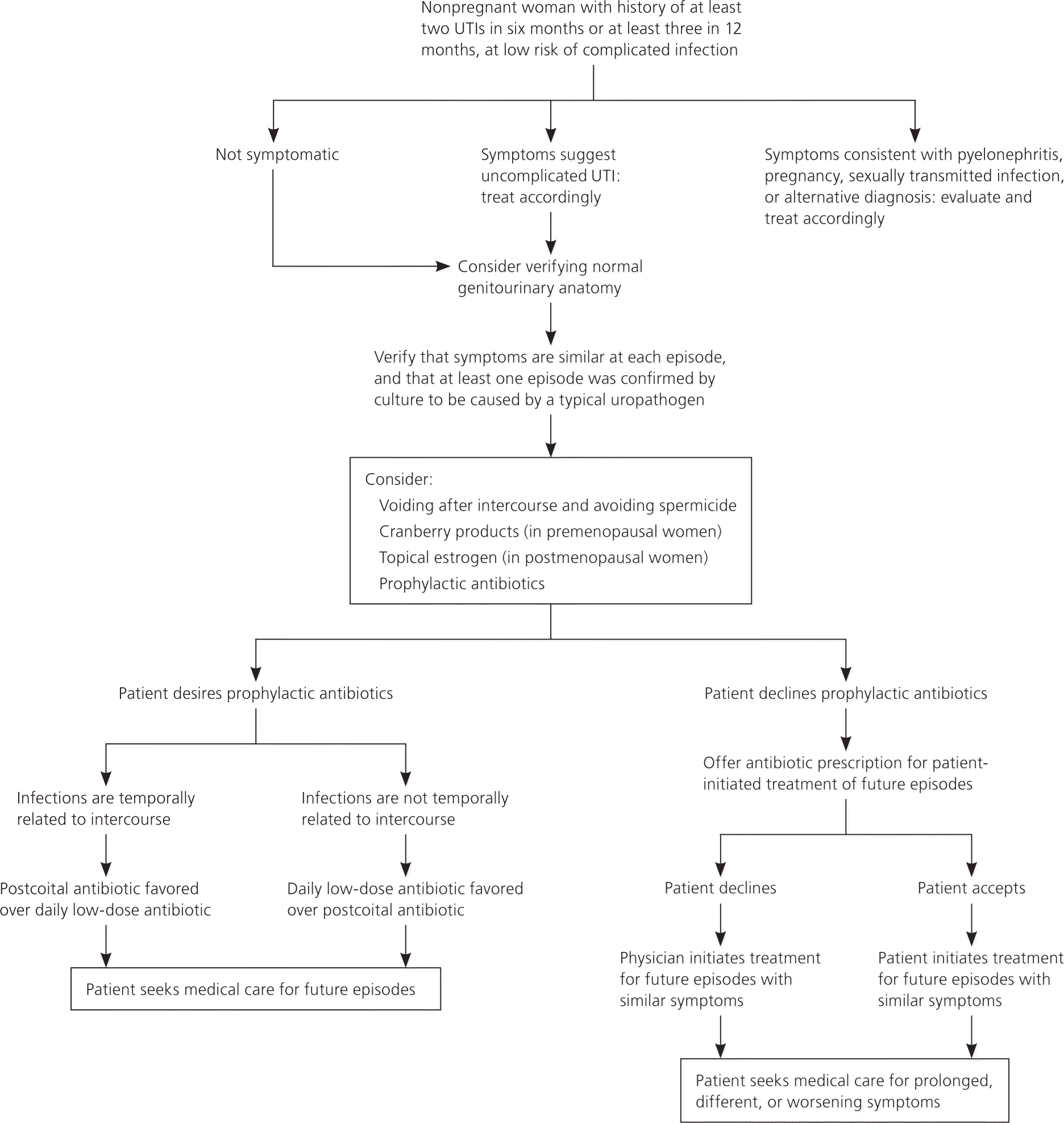
Patients who are candidates for prophylactic or self-initiated treatment should have at least one positive urine culture (at least 102 bacterial colonies per mL of a known urinary pathogen) while symptomatic to confirm concordance of symptoms with a true infection.3,5,11,15,17,22 Thereafter, repeat testing during recurrence of typical symptoms may increase cost and inconvenience for the patient, and subsequent benefit is unclear.11,12,17,23–25 However, repeat cultures should be obtained to establish resistance patterns in patients who have breakthrough UTIs while receiving prophylactic therapy.11,16 Cultures are warranted in patients with persistent UTI symptoms after 48 hours of antibiotic therapy, or with persistent symptomatic bacteriuria after two weeks of culture-directed antibiotic therapy because this may indicate a relapsed infection, which typically occurs because of antibiotic resistance or a persistent nidus of infection.3,11,13,18 Patients with persistent symptoms but negative cultures should be evaluated for a noninfectious cause of dysuria, such as interstitial cystitis or bladder cancer.3,11
The usefulness of pelvic examination in women with recurrent UTIs is limited; however, findings that predispose patients to complicated UTIs (e.g., cystocele, urethral diverticulum, fistula) may be detected.14,23 Imaging of the upper and lower urologic system with ultrasonography or computed tomography is typically unnecessary and should be guided by the presence of risk factors (Table 1).3,12,14,23 The diagnostic yield of cystoscopy suggesting anatomic abnormalities is less than 15%; therefore, routine cystoscopy is unwarranted.23,26–28 Cystoscopy in the setting of negative imaging findings is rarely diagnostic; therefore, noninvasive imaging should be completed first.23
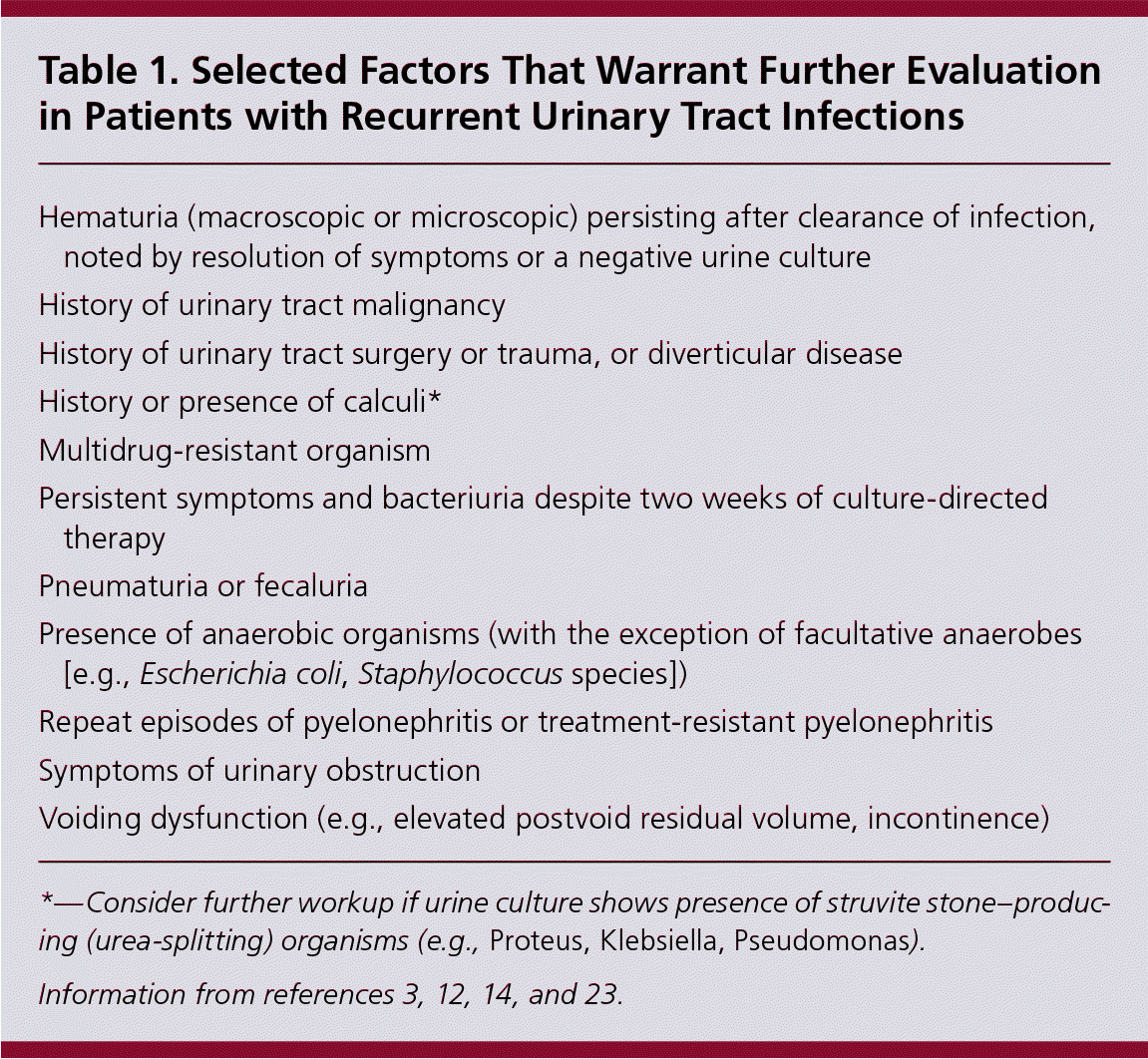
| Hematuria (macroscopic or microscopic) persisting after clearance of infection, noted by resolution of symptoms or a negative urine culture |
| History of urinary tract malignancy |
| History of urinary tract surgery or trauma, or diverticular disease |
| History or presence of calculi* |
| Multidrug-resistant organism |
| Persistent symptoms and bacteriuria despite two weeks of culture-directed therapy |
| Pneumaturia or fecaluria |
| Presence of anaerobic organisms (with the exception of facultative anaerobes [e.g., Escherichia coli, Staphylococcus species]) |
| Repeat episodes of pyelonephritis or treatment-resistant pyelonephritis |
| Symptoms of urinary obstruction |
| Voiding dysfunction (e.g., elevated postvoid residual volume, incontinence) |
Which Antibiotic Regimens Are Appropriate for Recurrent UTIs?
Uncomplicated infections can be treated with a three-day course of trimethoprim/sulfamethoxazole, a five-day course of nitrofurantoin, or a one-day course of fosfomycin (Monurol)1,29–32; these regimens are preferred to fluoroquinolones to minimize antibiotic resistance. Beta-lactams are less effective. Recommended regimens are the same for women with diabetes.
EVIDENCE SUMMARY
Patients with recurrent UTIs may be at higher risk of non–E. coli infection compared with those who have isolated acute cystitis.33 However, both disease processes are caused by similar pathogens and are treated according to local resistance patterns, patient factors, and drug availability (Table 2).1,3,5,11,12,21,23,29–32,34–36 Compared with longer treatment durations, three-day courses of bactericidal antimicrobials are associated with fewer adverse effects, improved treatment adherence, and similarly low risk of progression to pyelonephritis (less than 1%).11,29–31
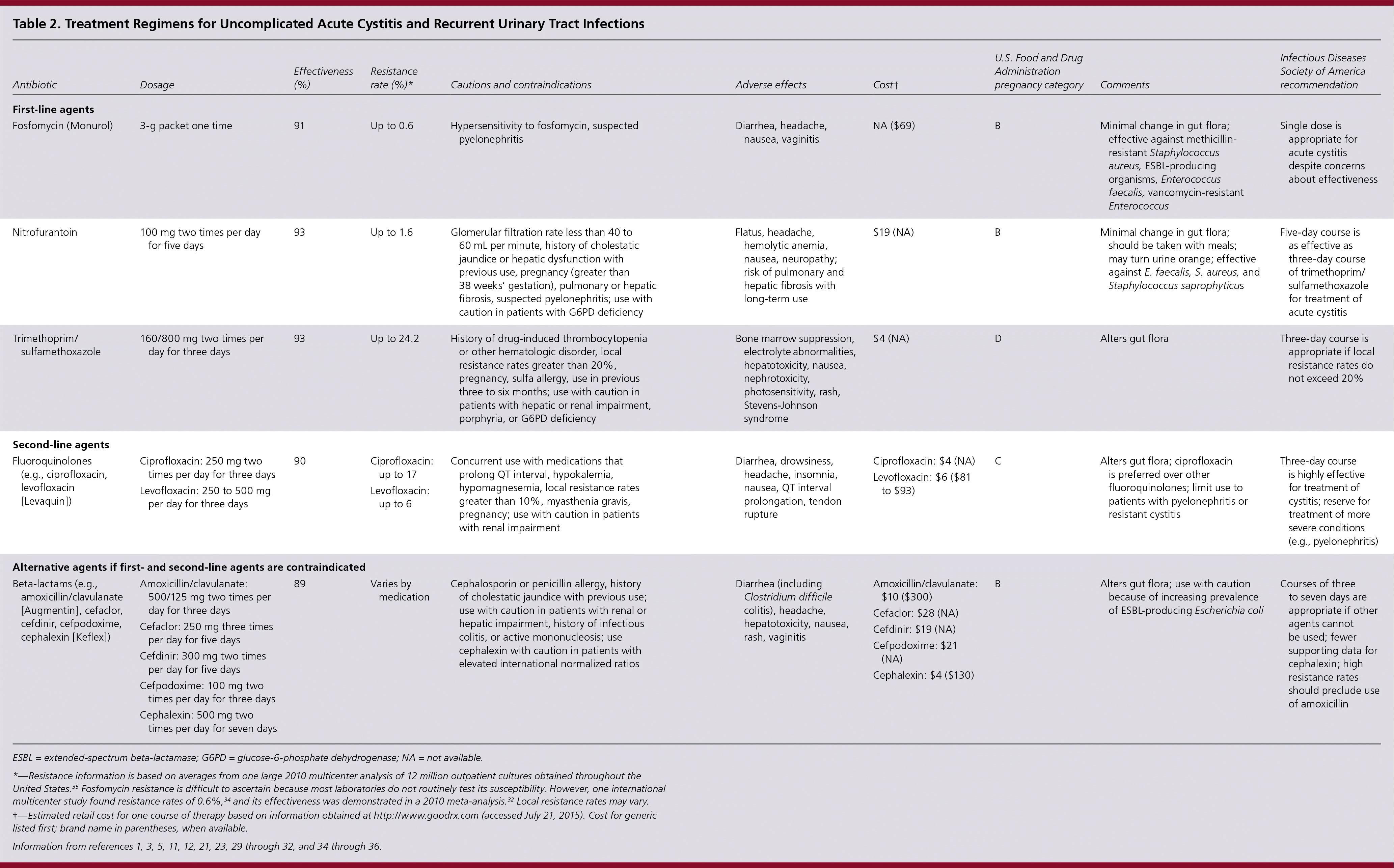
| Antibiotic | Dosage | Effectiveness (%) | Resistance rate (%)* | Cautions and contraindications | Adverse effects | Cost† | U.S. Food and Drug Administration pregnancy category | Comments | Infectious Diseases Society of America recommendation | |
|---|---|---|---|---|---|---|---|---|---|---|
| First-line agents | ||||||||||
| Fosfomycin (Monurol) | 3-g packet one time | 91 | Up to 0.6 | Hypersensitivity to fosfomycin, suspected pyelonephritis | Diarrhea, headache, nausea, vaginitis | NA ($69) | B | Minimal change in gut flora; effective against methicillin-resistant Staphylococcus aureus, ESBL-producing organisms, Enterococcus faecalis, vancomycin-resistant Enterococcus | Single dose is appropriate for acute cystitis despite concerns about effectiveness | |
| Nitrofurantoin | 100 mg two times per day for five days | 93 | Up to 1.6 | Glomerular filtration rate less than 40 to 60 mL per minute, history of cholestatic jaundice or hepatic dysfunction with previous use, pregnancy (greater than 38 weeks' gestation), pulmonary or hepatic fibrosis, suspected pyelonephritis; use with caution in patients with G6PD deficiency | Flatus, headache, hemolytic anemia, nausea, neuropathy; risk of pulmonary and hepatic fibrosis with long-term use | $19 (NA) | B | Minimal change in gut flora; should be taken with meals; may turn urine orange; effective against E. faecalis, S. aureus, and Staphylococcus saprophyticus | Five-day course is as effective as three-day course of trimethoprim/ sulfamethoxazole for treatment of acute cystitis | |
| Trimethoprim/ sulfamethoxazole | 160/800 mg two times per day for three days | 93 | Up to 24.2 | History of drug-induced thrombocytopenia or other hematologic disorder, local resistance rates greater than 20%, pregnancy, sulfa allergy, use in previous three to six months; use with caution in patients with hepatic or renal impairment, porphyria, or G6PD deficiency | Bone marrow suppression, electrolyte abnormalities, hepatotoxicity, nausea, nephrotoxicity, photosensitivity, rash, Stevens-Johnson syndrome | $4 (NA) | D | Alters gut flora | Three-day course is appropriate if local resistance rates do not exceed 20% | |
| Second-line agents | ||||||||||
| Fluoroquinolones (e.g., ciprofloxacin, levofloxacin [Levaquin]) |
| 90 |
| Concurrent use with medications that prolong QT interval, hypokalemia, hypomagnesemia, local resistance rates greater than 10%, myasthenia gravis, pregnancy; use with caution in patients with renal impairment | Diarrhea, drowsiness, headache, insomnia, nausea, QT interval prolongation, tendon rupture |
| C | Alters gut flora; ciprofloxacin is preferred over other fluoroquinolones; limit use to patients with pyelonephritis or resistant cystitis | Three-day course is highly effective for treatment of cystitis; reserve for treatment of more severe conditions (e.g., pyelonephritis) | |
| Alternative agents if first- and second-line agents are contraindicated | ||||||||||
| Beta-lactams (e.g., amoxicillin/clavulanate [Augmentin], cefaclor, cefdinir, cefpodoxime, cephalexin [Keflex]) |
| 89 | Varies by medication | Cephalosporin or penicillin allergy, history of cholestatic jaundice with previous use; use with caution in patients with renal or hepatic impairment, history of infectious colitis, or active mononucleosis; use cephalexin with caution in patients with elevated international normalized ratios | Diarrhea (including Clostridium difficile colitis), headache, hepatotoxicity, nausea, rash, vaginitis |
| B | Alters gut flora; use with caution because of increasing prevalence of ESBL-producing Escherichia coli | Courses of three to seven days are appropriate if other agents cannot be used; fewer supporting data for cephalexin; high resistance rates should preclude use of amoxicillin | |
Although UTIs in women with diabetes have historically been classified as complicated,3,23 new limited data suggest that causative pathogens and resistance rates are comparable to those of UTIs in women without diabetes.39–41 Two recent systematic reviews suggest that UTIs in women with diabetes should be treated in the same manner as those in women without diabetes unless risk factors for functionally or anatomically altered voiding are present.11,20
What Are the Benefits of Patient-Initiated Treatment?
Patient-initiated treatment lowers the cost of diagnosis, physician visits, and symptomatic days compared with physician-initiated treatment, and reduces antibiotic exposure compared with antibiotic prophylaxis.
EVIDENCE SUMMARY
No reduction in UTI episodes is achieved when patients initiate treatment. However, compared with prophylactic strategies or physician-initiated treatment, this approach seems to minimize the physiologic and financial cost of frequent antibiotic use, cost of diagnosis, number of physician visits, and number of symptomatic days, by limiting doses to symptomatic events.12,15,19,23–25,42
Which Prophylactic Regimens Are Recommended?
EVIDENCE SUMMARY
A large meta-analysis conducted in 2004 demonstrated that clinical recurrence of UTI is greatly reduced during antibiotic prophylaxis (relative risk [RR] = 0.15; 95% confidence interval [CI], 0.08 to 0.28; number needed to treat = 2).16 However, once prophylaxis was discontinued, patients reverted to pretreatment frequency of UTI.16
Clinicians should be mindful of local resistance patterns, patient factors, and drug availability when selecting the antimicrobial agent. Regimens have similar effectiveness, and all may lead to gastrointestinal upset, vaginal candidiasis, and rash.16
Compared with daily prophylaxis, postcoital prophylaxis reduces the total amount of antibiotics used without compromising effectiveness.5,10,11,16 This approach may be appropriate for patients with intercourse-associated UTIs, and may further limit antibiotic exposure in patients who have infrequent intercourse.3,5,10,11,16 Data suggest that intracellular bacterial communities coalesce as early as three hours after inoculation into the bladder; accordingly, antibiotics taken within two hours of intercourse may be optimal to prevent UTIs.5
The optimal duration of antibiotic prophylaxis is unknown. Based on consensus opinion and limited data, an initial six- to 12-month course should be offered.3,11,16 Small studies have shown effectiveness for up to five years, although long-term adverse effects such as antibiotic resistance and reversible pulmonary fibrosis from several years of nitrofurantoin use have been reported.3 Common dosing options for antibiotic prophylaxis are listed in Table 3.3,12,14,16,23
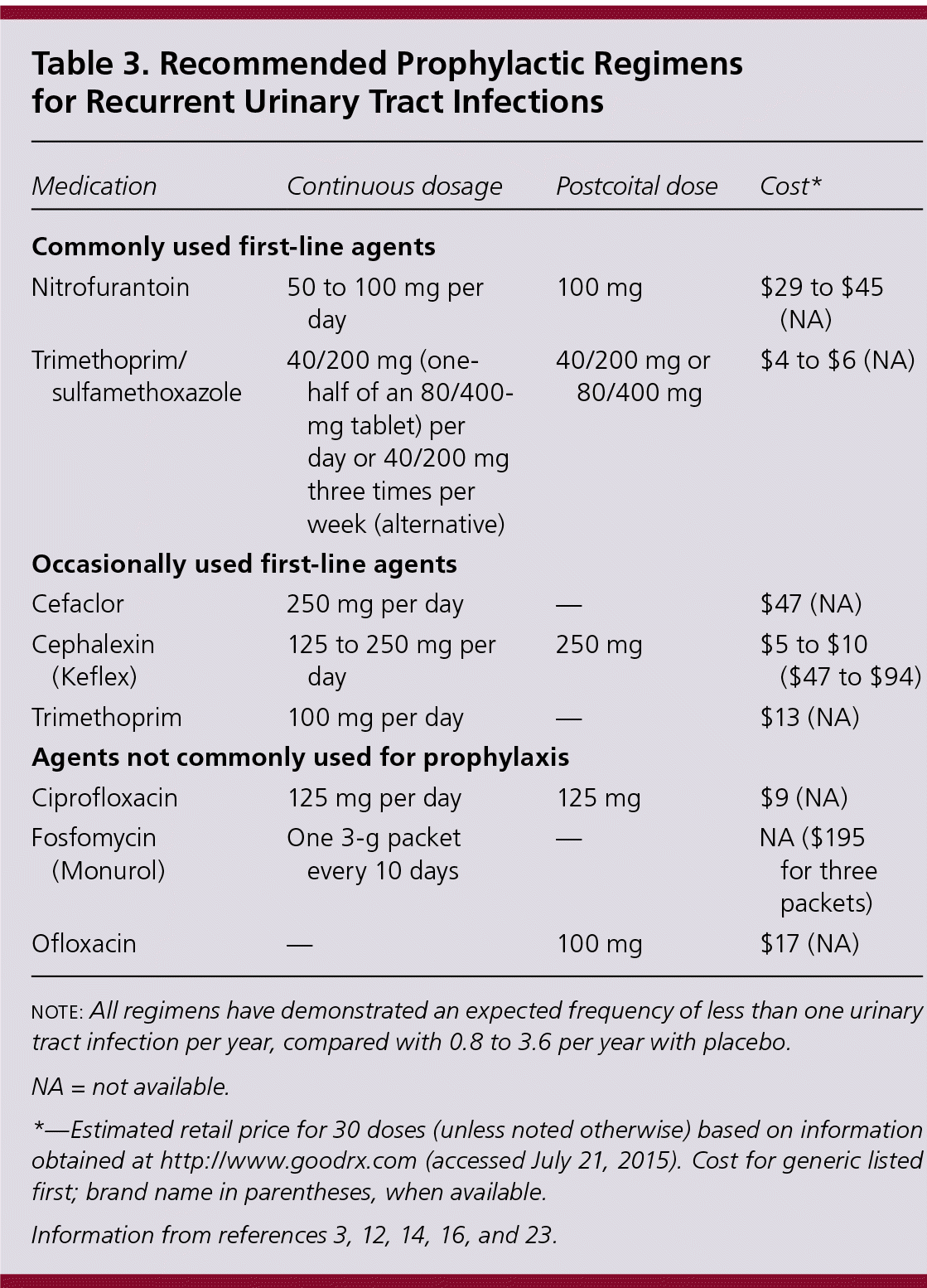
| Medication | Continuous dosage | Postcoital dose | Cost* |
|---|---|---|---|
| Commonly used first-line agents | |||
| Nitrofurantoin | 50 to 100 mg per day | 100 mg | $29 to $45 (NA) |
| Trimethoprim/sulfamethoxazole | 40/200 mg (one-half of an 80/400-mg tablet) per day or 40/200 mg three times per week (alternative) | 40/200 mg or 80/400 mg | $4 to $6 (NA) |
| Occasionally used first-line agents | |||
| Cefaclor | 250 mg per day | — | $47 (NA) |
| Cephalexin (Keflex) | 125 to 250 mg per day | 250 mg | $5 to $10 ($47 to $94) |
| Trimethoprim | 100 mg per day | — | $13 (NA) |
| Agents not commonly used for prophylaxis | |||
| Ciprofloxacin | 125 mg per day | 125 mg | $9 (NA) |
| Fosfomycin (Monurol) | One 3-g packet every 10 days | — | NA ($195 for three packets) |
| Ofloxacin | — | 100 mg | $17 (NA) |
Are There Alternative Treatment Strategies to Limit Antibiotic Use?
A short trial of an analgesic or anti-inflammatory medication for UTI symptoms can limit antibiotic use in willing patients when follow-up is assured. Delaying antibiotic treatment for urinary test results in patients with typical UTI symptoms is not recommended. Prophylaxis with a cranberry product in premenopausal women or topical estrogen therapy in postmenopausal women may limit UTI recurrences and thereby limit antibiotic use, although data about cranberry use are conflicting.
EVIDENCE SUMMARY
A 2009 meta-analysis suggested that symptomatic treatment alone, consisting of a one- to two-day course of a nonsteroidal anti-inflammatory drug (NSAID), is an option in patients with appropriate follow-up because up to one-third of infections resolve spontaneously within one week, with no difference in rates of progression to pyelonephritis.43 However, a recent small study showed a 60% decrease in antibiotic use but a slightly increased risk of pyelonephritis in patients who received ibu-profen.44 No recent or conclusive data exist to support the use of urinary tract analgesics such as phenazopyridine as single-agent treatment for UTI. However, they are generally low-risk medications and may provide analgesic alternatives to NSAIDs.45 Nonetheless, immediate antimicrobial therapy remains the quickest and most effective way to relieve symptoms and provides the best clinical outcomes.11
Cranberries contain proanthocyanidins, which may prevent adherence of E. coli to uroepithelial cells. Data are conflicting about the effectiveness of cranberry products for preventing recurrent UTI in premenopausal women.46–50 A 2012 meta-analysis demonstrated a decrease in UTI rates in women who received daily cranberry tablets (RR = 0.53; 95% CI, 0.33 to 0.83).48 However, a 2012 Cochrane review found insufficient evidence to recommend routine use of cranberry products for pro-phylaxis.50 They are generally a low-risk intervention and may prove to be another means to reduce UTI episodes and antibiotic use. Appropriate dosing and formulation of cranberry products have not been determined. Dosages of 36 to 72 mg per day are being tested in an ongoing clinical trial.49 Cranberry tablets seem to cause less gastroesophageal reflux and nausea than cranberry juice.46,47
In postmenopausal women, treatment of atrophic vaginitis with topical estrogen formulations may decrease rates of UTI recurrence through effects on vaginal flora.51,52 In a 2008 Cochrane review, women treated with topical estrogen had a 50% reduction in UTI recurrence.52 Oral estrogens are less effective and confer risks associated with systemic hormone replacement, and should not be used for this purpose.51,52
Data Sources: A PubMed search was completed using the MeSH function with the key phrase recurrent urinary tract infections combined with at least one of the following terms: women, non-pregnant, pre-menopausal and post-menopausal. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. Also searched were Essential Evidence Plus, the Cochrane Database of Systematic Reviews, the U.S. Preventive Services Task Force website, and relevant recommendations from the Infectious Diseases Society of America and the European Society of Clinical Microbiology and Infectious Diseases. Search dates: October 1, 2014, to February 14, 2016.
The authors thank Margaret Freiberg for her assistance in the preparation of the manuscript.
The opinions and assertions contained herein are the personal views of the authors and are not to be construed as official or as reflecting the views of the U.S. armed services or their medical departments.
