
A more recent article on vertebral compression fractures is available.
Am Fam Physician. 2016;94(1):44-50
Related letter: Lidocaine Patches Are No Better Than Placebo for Somatic Back Pain
Author disclosure: No relevant financial affiliations.
Vertebral compression fractures (VCFs) are the most common complication of osteoporosis, affecting more than 700,000 Americans annually. Fracture risk increases with age, with four in 10 white women older than 50 years experiencing a hip, spine, or vertebral fracture in their lifetime. VCFs can lead to chronic pain, disfigurement, height loss, impaired activities of daily living, increased risk of pressure sores, pneumonia, and psychological distress. Patients with an acute VCF may report abrupt onset of back pain with position changes, coughing, sneezing, or lifting. Physical examination findings are often normal, but can demonstrate kyphosis and midline spine tenderness. More than two-thirds of patients are asymptomatic and diagnosed incidentally on plain radiography. Acute VCFs may be treated with analgesics such as acetaminophen, nonsteroidal anti-inflammatory drugs, narcotics, and calcitonin. Physicians must be mindful of medication adverse effects in older patients. Other conservative therapeutic options include limited bed rest, bracing, physical therapy, nerve root blocks, and epidural injections. Percutaneous vertebral augmentation, including vertebroplasty and kyphoplasty, is controversial, but can be considered in patients with inadequate pain relief with nonsurgical care or when persistent pain substantially affects quality of life. Family physicians can help prevent vertebral fractures through management of risk factors and the treatment of osteoporosis.
Vertebral compression fractures (VCFs) are the most common complication of osteoporosis, affecting more than 700,000 Americans annually.1 Patients with VCFs account for 66,000 physician office visits and 45,000 to 70,000 hospitalizations each year, with one-half requiring skilled nursing facility care.2 Fracture risk increases with age; in the United States, four out of 10 white women older than 50 years will experience a hip, spine, or vertebral fracture in their lifetime.2 Women with one or more VCFs have a 1.2-fold greater age-adjusted mortality rate compared with women without fractures, with the risk of death increasing with the number of fractures.3 Fracture-related deaths occur after the fracture, often from pulmonary disease or cancer.3,4 Furthermore, patients report a lower quality of life at 12 and 24 months after a fracture.2 The estimated direct annual health care cost of managing osteoporotic spine and hip fractures is $10 billion to $15 billion.5
WHAT IS NEW ON THIS TOPIC: VERTEBRAL COMPRESSION FRACTURES
Two randomized controlled trials comparing vertebroplasty with a sham procedure in patients with acute or chronic vertebral compression fractures found no benefit in pain reduction, function, or quality of life.
Patients with vertebral compression fractures should not undergo vertebroplasty unless they continue to have debilitating pain or substantial functional limitations after at least three weeks of conservative therapy.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| A trial of conservative therapy should be offered to patients with vertebral compression fractures. | C | 13, 17, 18, 21 |
| Percutaneous vertebral augmentation can be considered in patients who have inadequate pain relief with nonsurgical care or when persistent pain substantially affects quality of life. | C | 13, 15, 17, 21 |
| Patients with vertebral compression fractures should be evaluated for osteoporosis, and preventive therapy should be initiated if necessary. | C | 6, 22, 41 |
Risk Factors
Risk factors for VCFs include osteopenia, osteoporosis, older age, a history of VCFs or falls, inactivity, use of corticosteroids (more than 5 mg daily for three months) or other medications, weight less than 117 lb (53.1 kg), female sex, consumption of more than two alcoholic drinks per day in women or more than three per day in men, smoking, vitamin D deficiency, and depression.6–9
Clinical Presentation
More than two-thirds of patients with VCFs are asymptomatic, and are diagnosed incidentally.10 Symptomatic patients may present with back pain and fracture demonstrated on radiography, most commonly between T8 and L4.11 Patients with an acute fracture may report abrupt onset of pain with position changes, coughing, sneezing, or lifting.8,12,13 Physical examination findings are often normal, but may demonstrate kyphosis and midline spine tenderness.8,12 Chronic VCF may present with loss of height in addition to kyphosis. Complications include bone loss, muscle weakness, pressure sores, ileus, urinary retention, impaired respiratory function, venous thromboembolism, and spinal cord compression.12,14–17
Evaluation
The physical examination should include a neurologic assessment. Compression fractures are typically diagnosed by lateral radiography of the vertebral column, with or without anteroposterior views.19 Radiographic criteria for VCFs include a decrease in vertebral body height of at least 20% or a 4-mm reduction from baseline height.14 The classic radiographic finding is an anterior wedge fracture8,12,19 (Figure 18 ).

Magnetic resonance imaging (MRI) can help distinguish benign from malignant fractures and determine the timing of the fracture, because recent fractures display edema.14,15 MRI or computed tomography is useful for identifying suspected retropulsion, fractures extending to the posterior column, and spinal cord involvement.14,15 Computed tomography or MRI should also be considered in patients who do not improve with conservative care and in those with progressive symptoms.8,14,15,17 Dual-energy x-ray absorptiometry should be performed soon after the diagnosis of a VCF to evaluate for osteoporosis and determine disease severity.6,14,15
If secondary osteoporosis is suspected (e.g., in a younger patient or in one with symptoms of hypercalcemia or anemia), laboratory evaluation may include a complete blood count; complete metabolic panel with liver function testing; and measurement of erythrocyte sedimentation rate and thyroid-stimulating hormone, 25-hydroxyvitamin D, parathyroid hormone, and C-reactive protein levels.6 Blood cultures are recommended when infection is suspected. Serum and urine protein electrophoresis should be performed if multiple myeloma is suspected. There is a high prevalence of low testosterone levels in younger men with osteoporosis and low-trauma fractures, and measurement of the testosterone level may be considered in these patients.20
Treatment
Goals of treatment include pain relief, restoration of function, and prevention of future fractures.12,14 Treatment of VCFs should begin with discussion of patient goals and risks, and benefits of conservative care vs. percutaneous vertebral augmentation. Patients who wish to pursue conservative treatment will have more than a 50% chance of sufficient pain reduction, most of which occurs by three months.21 A study of 259 patients with VCFs showed that patients who had pain relief and reduced disability with three weeks of conservative therapy had a 95% chance of maintaining these improvements for up to 12 months.21
CONSERVATIVE CARE
Early mobility should be encouraged as soon as it can be tolerated. Bed rest is sometimes recommended as part of initial management if pain is intolerable, but it can lead to loss of bone mass and muscle strength, pressure sores, and deep venous thrombosis.17 The American Academy of Orthopaedic Surgeons (AAOS) found inconclusive evidence regarding the benefits of bed rest in the treatment of VCFs.22
Medications. Nonsteroidal anti-inflammatory drugs, acetaminophen, narcotics, lidocaine patches, and muscle relaxants are commonly used for pain relief.7,8,12,14 Medications facilitate patient mobility and participation in physical therapy, and should be tapered slowly as pain improves.12 The AAOS found inconclusive evidence to support specific analgesics for acute VCF pain.22 In neurologically intact patients with VCF, calcitonin significantly reduces pain and facilitates earlier mobilization for up to four weeks.22–25 Table 1 reviews common medications used to treat VCFs.26 Physicians should be mindful of medication adverse effects in older patients.
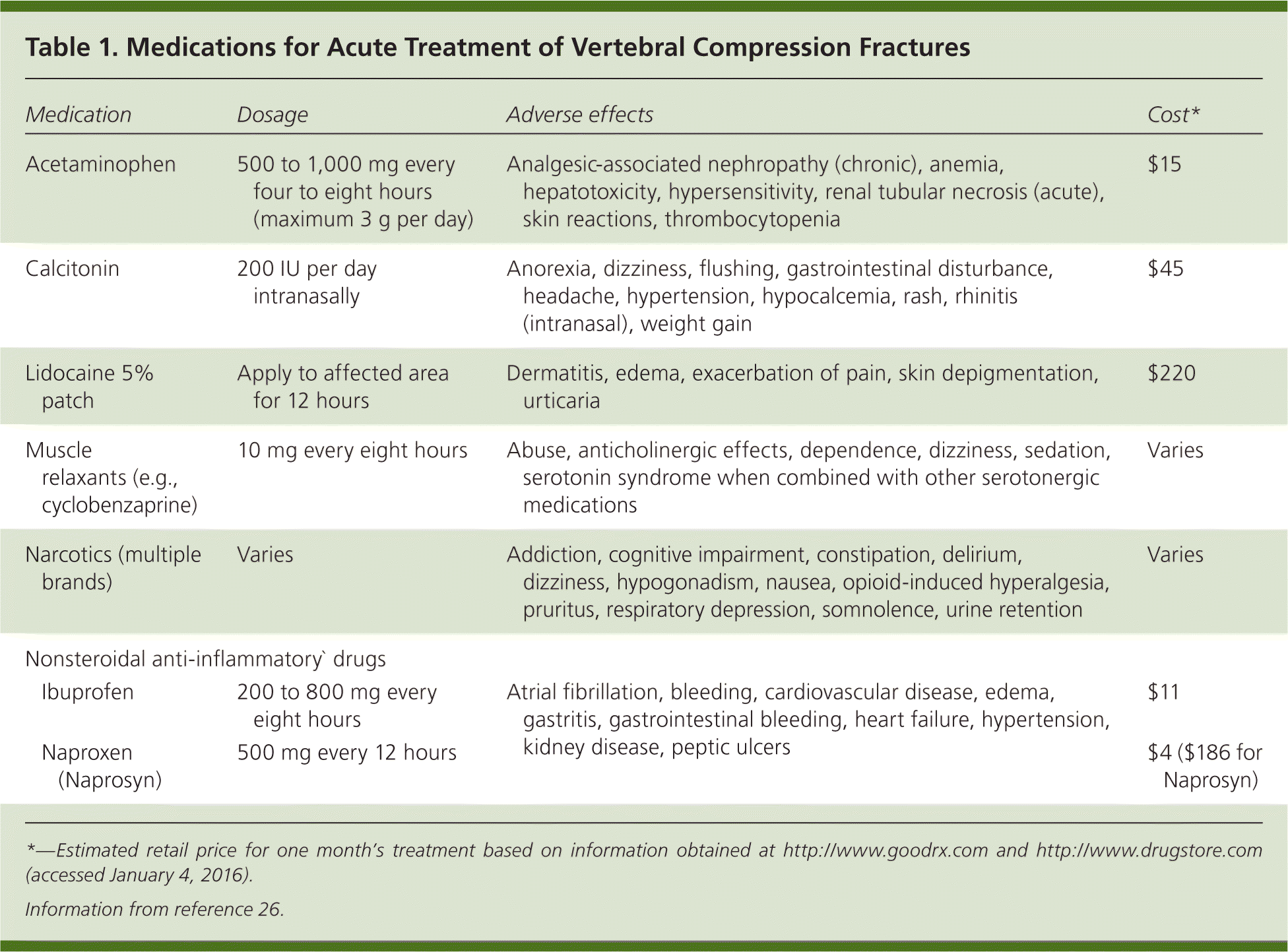
| Medication | Dosage | Adverse effects | Cost* | |
|---|---|---|---|---|
| Acetaminophen | 500 to 1,000 mg every four to eight hours (maximum 3 g per day) | Analgesic-associated nephropathy (chronic), anemia, hepatotoxicity, hypersensitivity, renal tubular necrosis (acute), skin reactions, thrombocytopenia | $15 | |
| Calcitonin | 200 IU per day intranasally | Anorexia, dizziness, flushing, gastrointestinal disturbance, headache, hypertension, hypocalcemia, rash, rhinitis (intranasal), weight gain | $45 | |
| Lidocaine 5% patch | Apply to affected area for 12 hours | Dermatitis, edema, exacerbation of pain, skin depigmentation, urticaria | $220 | |
| Muscle relaxants (e.g., cyclobenzaprine) | 10 mg every eight hours | Abuse, anticholinergic effects, dependence, dizziness, sedation, serotonin syndrome when combined with other serotonergic medications | Varies | |
| Narcotics (multiple brands) | Varies | Addiction, cognitive impairment, constipation, delirium, dizziness, hypogonadism, nausea, opioid-induced hyperalgesia, pruritus, respiratory depression, somnolence, urine retention | Varies | |
| Nonsteroidal anti-inflammatory drugs | ||||
| Ibuprofen | 200 to 800 mg every eight hours | Atrial fibrillation, bleeding, cardiovascular disease, edema, gastritis, gastrointestinal bleeding, heart failure, hypertension, kidney disease, peptic ulcers | $11 | |
| Naproxen (Naprosyn) | 500 mg every 12 hours | $4 ($186 for Naprosyn) | ||
Bracing. Although bracing is commonly prescribed for six to eight weeks after a VCF, the evidence is limited.14,22 A small study of thoracolumbar bracing improved posture, strength, and quality of life.27 In another study, disability scores were not significantly improved in patients who wore a rigid brace or soft brace compared with those who did not wear a brace.28 Potential benefits of pain reduction should be balanced against the risks of muscle atrophy and skin complications.12,13
Physical Therapy and Exercise. Physical therapy is likely to be useful in patients with VCFs and osteoporosis.12,14,29 Home exercise programs have a more limited evidence base, with some small trials demonstrating pain reduction, improved balance, and quality of life.22,30 Back extensor strengthening can improve strength and bone density, and reduce the risk of future VCFs.31 Exercise is beneficial for all patients with osteoporosis.6
Nerve Root Blocks. The AAOS gives a weak recommendation for the use of L2 nerve blocks for temporary pain reduction in patients with VCFs.22 Patients undergoing L2 selective nerve blocks have reduced pain for up to two weeks, with effects dissipating by one month.22 Patients with radicular pain may benefit from nerve root blocks or epidural injections.14 Family physicians should counsel patients to weigh the benefits of temporary pain relief against the risks of the procedure.
VERTEBROPLASTY AND KYPHOPLASTY
Percutaneous vertebral augmentation, including vertebroplasty or kyphoplasty, can be considered in patients with inadequate pain relief from nonsurgical care, or when persistent pain substantially affects quality of life13,15,17,18,21; however, recent studies have questioned their effectiveness.32–34 Vertebroplasty entails injecting liquid cement into a collapsed vertebral body through a needle inserted transpedicularly. Kyphoplasty involves percutaneously injecting a balloon into the vertebral body, inflating it to restore vertebral height, and injecting cement to reduce pain.15,17 Complications include extravasation of cement (more common with vertebroplasty), embolism, neurologic injury, bleeding, hematoma, infection, and an increased risk of VCFs at other levels.13,15,17
In 2010, the AAOS strongly recommended against vertebroplasty in neurologically intact patients with VCFs.22 Two randomized controlled trials comparing vertebroplasty with a sham procedure in patients with acute or chronic VCF found no benefit in pain reduction, function, or quality of life.32,33 In contrast, a 2013 meta-analysis of six randomized controlled trials (including those that found no benefits) found that vertebroplasty provided better pain relief, functionality, and quality of life compared with conservative care at 12 weeks, six months, and 12 months.35
A 2014 consensus statement from several U.S. and Canadian neurosurgical and radiologic groups supports offering vertebroplasty and kyphoplasty to patients receiving medical therapy who are unable to ambulate after 24 hours of treatment, who have pain intense enough to prevent participation in physical therapy, or who have adverse effects from analgesics.17 Potential benefits must be evaluated against the failure of percutaneous vertebral augmentation to improve mortality or major medical outcomes and the increased health care utilization and complications associated with the procedures. Mortality benefits reported in studies of percutaneous vertebral augmentation may be linked to selection bias caused by the exclusion of patients at high risk of complications.34
Based on current evidence, most patients should not undergo percutaneous vertebral augmentation unless they present with acute MRI-confirmed fracture and debilitating pain or substantial functional limitations despite conservative therapy for at least three weeks.
Prevention
Optimal treatment of patients with VCFs includes prevention of additional fractures and treatment of osteoporosis. Family physicians can encourage weight-bearing and muscle-strengthening exercise, smoking cessation, and avoidance of excessive alcohol consumption; and assess the risk of falls.6
Screening for osteoporosis can identify patients who are most likely to benefit from treatment to reduce the likelihood of VCFs. The Institute of Medicine recommends adequate intake of calcium (1,000 mg per day for men 50 to 70 years of age, and 1,200 mg per day for women 51 years and older and men 71 years and older) and vitamin D (600 IU per day up to 70 years of age, 800 IU per day after 70 years of age).6,37 However, the U.S. Preventive Services Task Force found insufficient evidence to recommend more than 400 IU per day of supplemental vitamin D or more than 1,000 mg per day of calcium for primary prevention of fractures in non-institutionalized postmenopausal women, and they recommend against supplementation with lower amounts because of proven lack of benefit.38,39
Patients with VCFs have a fivefold increased risk of subsequent VCFs and a two- to threefold increased risk of fractures at other sites.6 Those with a hip fracture or VCF should be evaluated for osteoporosis. Patients with a T-score of −2.5 or lower at the femoral neck, total hip, or lumbar spine; a T-score of −1 to −2.4 at the femoral neck or lumbar spine; and a 10-year probability of hip fracture of 3% or more; or a 10-year probability of a major osteoporosis-related fracture (clinical vertebral, hip, forearm, or proximal humerus fracture) of 20% or more should receive treatment.6
Medications approved by the U.S. Food and Drug Administration for the treatment and prevention of osteoporosis include bisphosphonates, calcitonin, estrogen, selective estrogen receptor modulators, parathyroid hormone, and receptor activator of nuclear factor kappa-B ligand inhibitors6 (Table 23,8,34,36,37,39–41 ). Multiple bisphosphonates are approved for the primary and secondary prevention of VCFs.6,22,40,41 Although estrogen therapy has been approved for the prevention of osteoporosis, it should be considered only after nonestrogen treatments have been tried.6 The anabolic agent teriparatide (Forteo) reduces the risk of subsequent VCFs, although it is expensive and must be administered by daily subcutaneous injection.6,42 Additionally, denosumab (Prolia) leads to a relative decrease in new VCFs compared with placebo in postmenopausal women with osteoporosis. Denosumab can be used as an alternative to other therapies for the primary prevention of VCFs in postmenopausal women with osteoporosis.43

| Medication | Dosage | Relative risk reduction in vertebral fractures | Mechanism of action | Used for | |
|---|---|---|---|---|---|
| Bisphosphonates | Antiresorptives; inhibit osteoclasts by inducing apoptosis | Prevention and treatment | |||
| Alendronate (Fosamax) | 5 mg per day (prevention) or 35 mg per week (treatment) | 50% in patients with history of fracture; 48% in those without | |||
| Ibandronate (Boniva) | 150 mg per month | 50% | |||
| Risedronate (Actonel) | 5 mg per day or 35 mg per week | 41% to 49% | |||
| Zoledronic acid (Reclast) | 5 mg intravenously every two years (prevention) or 5 mg intravenously per year (treatment) | 70% | |||
| Calcitonin | 50 to 100 IU per day intramuscularly or 200 IU per day intranasally | 30% in patients with history of fracture | Antiresorptive | Treatment | |
| Calcium | 1,000 to 1,200 mg per day | — | — | Prevention | |
| Estrogen | 0.3 mg or 0.625 mg per day via pill or patch | 34% | Antiresorptive | Prevention | |
| Estrogen agonist/antagonist (raloxifene [Evista]) | 60 mg per day | 30% in patients with history of fracture; 55% in those without | Antiresorptive | Prevention and treatment | |
| Parathyroid hormone (teriparatide [Forteo]) | 20 mcg per day subcutaneously for up to 24 months | 65% | Maintains osteocytes, increases intestinal calcium absorption, decreases urinary calcium, increases vitamin D production | Treatment | |
| RANKL inhibitor (denosumab [Prolia]) | 60 mg per day subcutaneously every six months | 68% | Blocks formation, function, and survival of osteoclasts | Treatment | |
| Vitamin D | 800 to 1,000 IU per day | — | — | Prevention | |
Data Sources: Medline and Essential Evidence Plus searches were completed using the following MeSH terms: fractures; fractures, compression; spinal cord compression; spinal fractures. MeSH subheadings included: drug therapy, prevention & control, rehabilitation, therapy. The following key words were used: vertebral compression fracture, conservative, nonoperative, nonsurgical. The search included randomized controlled trials, meta-analyses, clinical trials, systematic reviews, clinical practice guidelines, evidence-based medicine, and review articles. Search dates: January 1996 through December 2014.
The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the U.S. Air Force or the Department of Defense.
