
Am Fam Physician. 2017;95(6):373-383A
Patient information: See related handout on multiple myeloma, written by the authors of this article.
Author disclosure: No relevant financial affiliations.
Multiple myeloma accounts for 1.6% of all cancer cases and approximately 10% of hematologic malignancies in the United States. In 2015, an estimated 28,850 new cases of multiple myeloma were diagnosed in the United States, and the disease caused more than 11,000 deaths. Patients older than 65 years account for 85% of those diagnosed with multiple myeloma, and there is a twofold increased incidence in blacks compared with whites. Patients may present with bone pain or with symptoms that are often nonspecific, such as nausea, vomiting, malaise, weakness, recurrent infections, and weight loss. Many patients present with only laboratory abnormalities, such as anemia, renal disease, and elevated protein levels. The diagnosis of multiple myeloma requires increased numbers of immature, abnormal, or atypical plasma cells in the bone marrow; a monoclonal protein in the serum or urine; or characteristic bone lesions. The diagnostic workup in a patient with suspected multiple myeloma should include a complete blood count with differential; serum chemistries; creatinine, lactate dehydrogenase, and beta2-microglobulin tests; immunoglobulin studies; skeletal survey; and bone marrow evaluation. Initiation of chemotherapy and assessment of eligibility for autologous stem cell transplantation require referral to an oncologist. Most patients with multiple myeloma will receive thromboprophylaxis, bisphosphonate therapy, and prophylaxis against infection at some point in their treatment. Family physicians play a role in assessing these patients for infection, adverse treatment effects, and renal and thrombotic complications, and in managing issues related to pain, nutrition, and psychosocial support.
Multiple myeloma is a malignancy of plasma cells; these cells accumulate in bone marrow and overproduce a monoclonal protein. Plasma cell malignancies include a spectrum of diseases, from monoclonal gammopathy of undetermined significance (MGUS) to smoldering multiple myeloma (SMM), clinical multiple myeloma, and, rarely, plasma cell leukemia. The disease process is insidious, with end-organ damage occurring over years.1,2
WHAT IS NEW ON THIS TOPIC: MULTIPLE MYELOMA
In 2014, the International Myeloma Working Group revised the diagnostic criteria for multiple myeloma. These new criteria add myeloma-defining events, the presence of any one of which is sufficient to diagnose multiple myeloma.
Intravenous zoledronic acid (Reclast) or pamidronate is recommended for all patients with multiple myeloma who are receiving treatment, regardless of the presence of bone lesions.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| The diagnostic workup for suspected multiple myeloma should include a complete blood count with differential; serum chemistries; measurement of creatinine, lactate dehydrogenase, and beta2-microglobulin levels; immunoglobulin studies; a skeletal survey; and bone marrow evaluation. | C | 10–12 |
| Patients with multiple myeloma should be evaluated by an oncologist to determine if they are a candidate for autologous stem cell transplantation; this should include assessing comorbid conditions and functional status, which may be defined upon referral. | C | 2, 6, 15, 16 |
| Patients with multiple myeloma should receive bisphosphonate therapy (i.e., zoledronic acid [Reclast] or pamidronate) when first diagnosed. | A | 37, 38 |
| Patients with multiple myeloma should receive thromboprophylaxis when first diagnosed. | C | 41, 42 |
Epidemiology and Pathophysiology
Multiple myeloma accounts for 1.6% of all cancer cases and approximately 10% of hematologic malignancies in the United States.3 In 2015, there were an estimated 28,850 new cases of multiple myeloma diagnosed in the United States and more than 11,000 related deaths.3 The median age of presentation is 70 years; only 15% of patients diagnosed with multiple myeloma are younger than 65 years. Blacks have a twofold higher incidence compared with whites and present at a younger age.4
Cytogenetic abnormalities are detected in 90% of the plasma cells in patients with multiple myeloma, and multistep genetic alterations lead to the progression from MGUS to multiple myeloma in some persons.1,2 The monoclonal protein produced by these plasma cells is an abnormal immunoglobulin (immunoglobulin G [IgG], IgM, or IgA, or, rarely, IgE or IgD) or light chain protein (kappa or lambda), either of which causes hyperviscosity and end-organ damage. Invasive bone lesions can cause pathologic fractures, bone pain, osteoporosis, and hypercalcemia. Bone marrow invasion leads to anemia, and immunologic changes cause recurrent infections.1,5
Patients with MGUS develop multiple myeloma at a rate of approximately 1% per year. SMM progression occurs at a rate of 10% per year for the first five years, then at a lower rate. Risk factors for disease progression of these conditions include non-IgG subtype, higher levels of monoclonal protein, abnormal free light chain ratio, and certain gene alterations.2,6,7
Clinical Presentation and Evaluation
In asymptomatic patients, multiple myeloma is most likely to be identified through laboratory abnormalities such as hypercalcemia, anemia, or proteinuria.8 Patients may present with nonspecific symptoms, such as nausea, vomiting, malaise, weakness, recurrent infections, or weight loss. Symptoms of bone disease (e.g., pain from fracture or plasmacytoma, spinal cord compression), peripheral neuropathy, or hyperviscosity (e.g., dyspnea, transient ischemic attack, retinal hemorrhage, deep venous thrombosis) can occur. Anemia is present in nearly all patients with multiple myeloma at some point in the disease (Table 1).9 A detailed history focused on symptoms should be taken when any of these results or symptoms are reported.
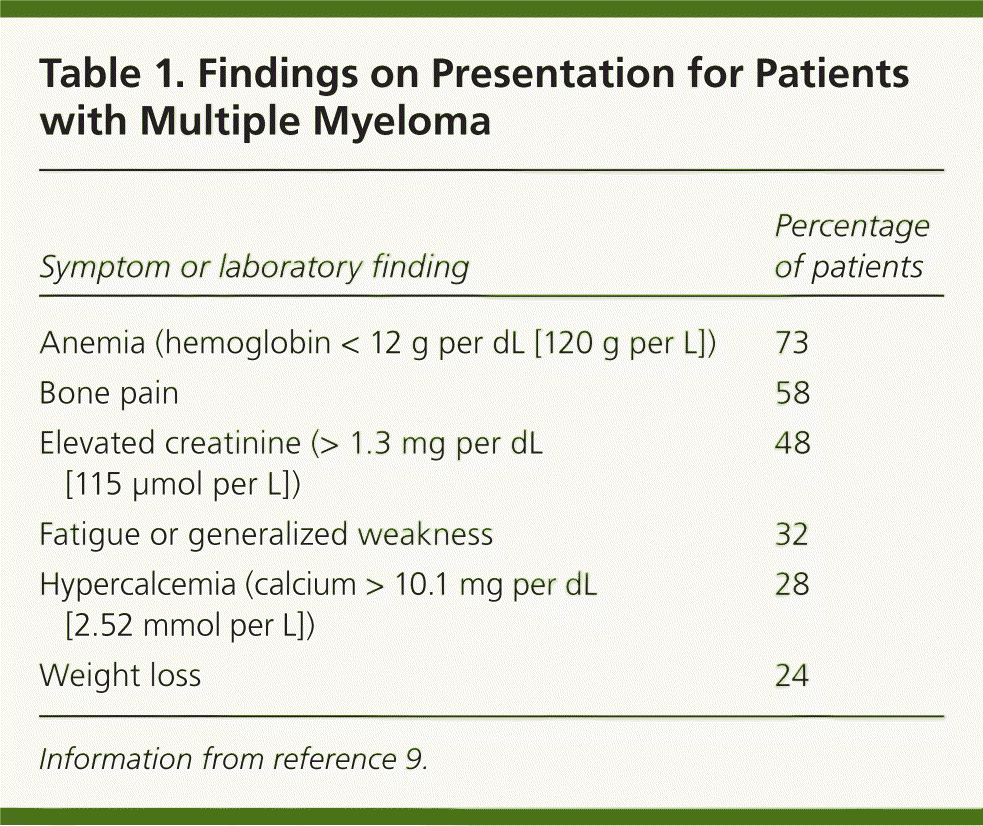
| Symptom or laboratory finding | Percentage of patients |
|---|---|
| Anemia (hemoglobin < 12 g per dL [120 g per L]) | 73 |
| Bone pain | 58 |
| Elevated creatinine (> 1.3 mg per dL [115 μmol per L]) | 48 |
| Fatigue or generalized weakness | 32 |
| Hypercalcemia (calcium > 10.1 mg per dL [2.52 mmol per L]) | 28 |
| Weight loss | 24 |
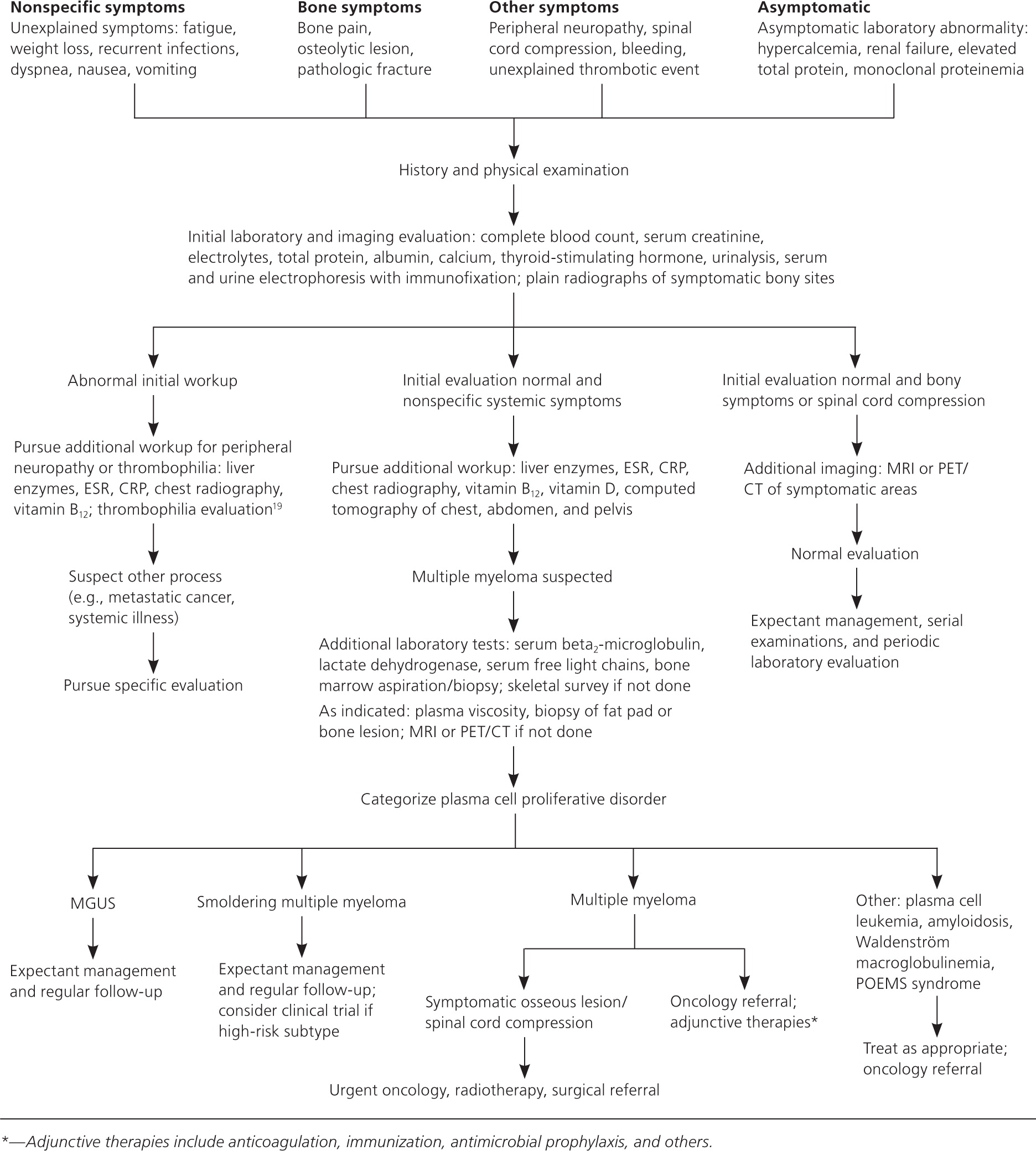
Physical examination is primarily helpful for identifying other symptom causes. Most patients with multiple myeloma have normal physical examination findings on presentation.9 Laboratory evaluation in a patient with suspected multiple myeloma has the highest diagnostic yield. Initial and follow-up tests are discussed in Table 210–12 and Figure 1.1,2,6,12–19
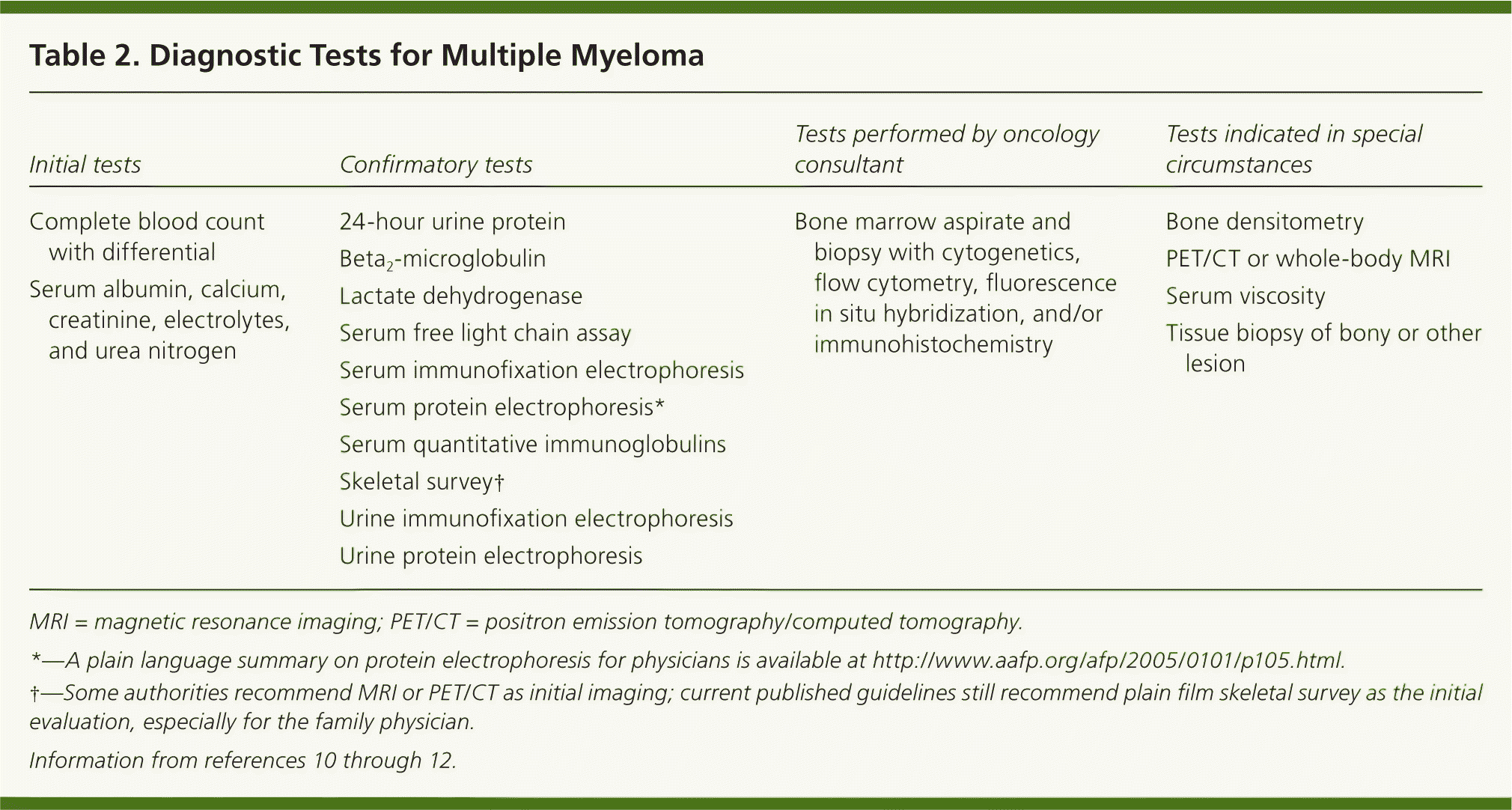
| Initial tests | Confirmatory tests | Tests performed by oncology consultant | Tests indicated in special circumstances |
|---|---|---|---|
|
|
|
Differential Diagnosis
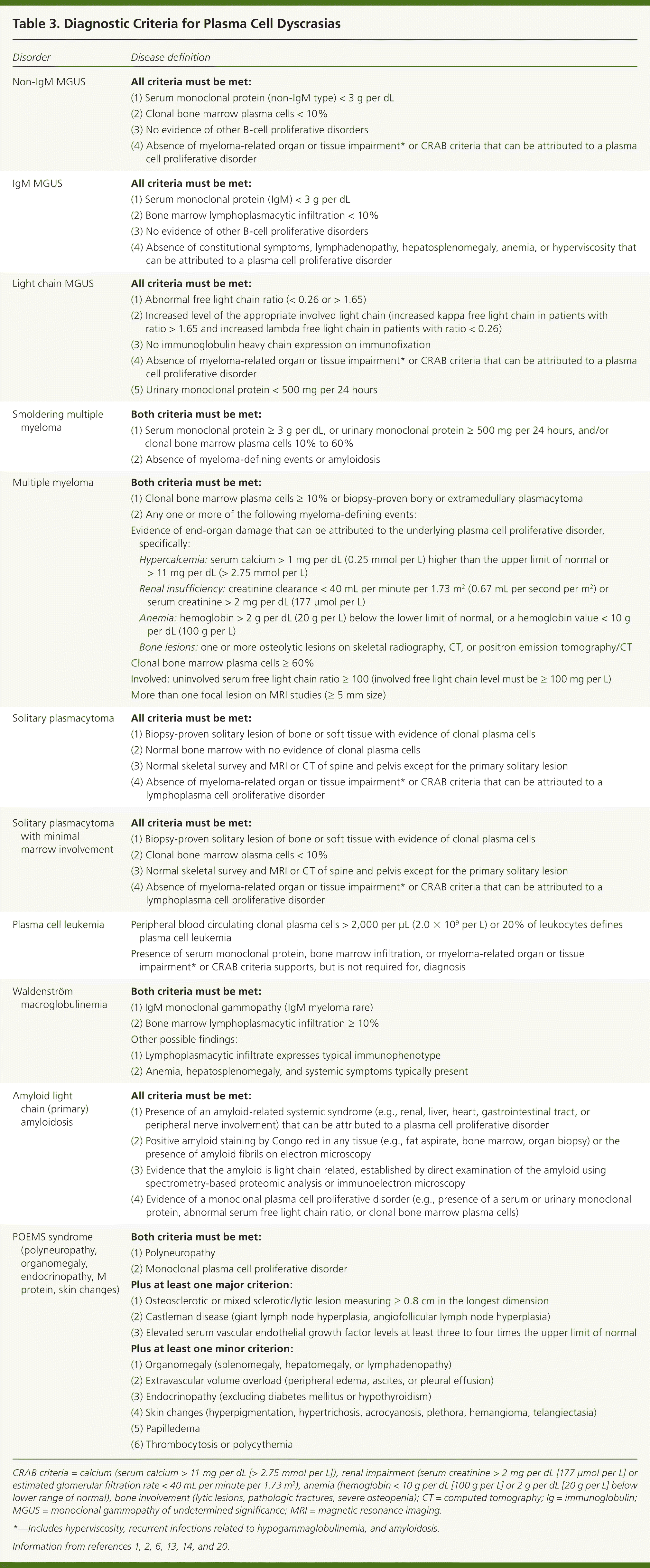
The differential diagnosis of multiple myeloma usually involves the spectrum of plasma cell proliferative disorders shown in Table 3.1,2,6,13,14,20 A full evaluation will help classify where a patient falls in this spectrum. The differential diagnosis of bone lesions includes primary or metastatic cancer, benign bone lesions, osteoporotic compression fracture, and other bone conditions.21,22 The full differential diagnosis for patients presenting with fatigue, unexplained weight loss, or hypercalcemia is broad and beyond the scope of this article.23–25
| Disorder | Disease definition | |
|---|---|---|
| Non-IgM MGUS | All criteria must be met: | |
| (1) Serum monoclonal protein (non-IgM type) < 3 g per dL | ||
| (2) Clonal bone marrow plasma cells < 10% | ||
| (3) No evidence of other B-cell proliferative disorders | ||
| (4) Absence of myeloma-related organ or tissue impairment* or CRAB criteria that can be attributed to a plasma cell proliferative disorder | ||
| IgM MGUS | All criteria must be met: | |
| (1) Serum monoclonal protein (IgM) < 3 g per dL | ||
| (2) Bone marrow lymphoplasmacytic infiltration < 10% | ||
| (3) No evidence of other B-cell proliferative disorders | ||
| (4) Absence of constitutional symptoms, lymphadenopathy, hepatosplenomegaly, anemia, or hyperviscosity that can be attributed to a plasma cell proliferative disorder | ||
| Light chain MGUS | All criteria must be met: | |
| (1) Abnormal free light chain ratio (< 0.26 or > 1.65) | ||
| (2) Increased level of the appropriate involved light chain (increased kappa free light chain in patients with ratio > 1.65 and increased lambda free light chain in patients with ratio < 0.26) | ||
| (3) No immunoglobulin heavy chain expression on immunofixation | ||
| (4) Absence of myeloma-related organ or tissue impairment* or CRAB criteria that can be attributed to a plasma cell proliferative disorder | ||
| (5) Urinary monoclonal protein < 500 mg per 24 hours | ||
| Smoldering multiple myeloma | Both criteria must be met: | |
| (1) Serum monoclonal protein ≥ 3 g per dL, or urinary monoclonal protein ≥ 500 mg per 24 hours, and/or clonal bone marrow plasma cells 10% to 60% | ||
| (2) Absence of myeloma-defining events or amyloidosis | ||
| Multiple myeloma | Both criteria must be met: | |
| (1) Clonal bone marrow plasma cells ≥ 10% or biopsy-proven bony or extramedullary plasmacytoma | ||
| (2) Any one or more of the following myeloma-defining events: | ||
| Evidence of end-organ damage that can be attributed to the underlying plasma cell proliferative disorder, specifically: | ||
| Hypercalcemia: serum calcium > 1 mg per dL (0.25 mmol per L) higher than the upper limit of normal or > 11 mg per dL (> 2.75 mmol per L) | ||
| Renal insufficiency: creatinine clearance < 40 mL per minute per 1.73 m2 (0.67 mL per second per m2) or serum creatinine > 2 mg per dL (177 μmol per L) | ||
| Anemia: hemoglobin > 2 g per dL (20 g per L) below the lower limit of normal, or a hemoglobin value < 10 g per dL (100 g per L) | ||
| Bone lesions: one or more osteolytic lesions on skeletal radiography, CT, or positron emission tomography/CT | ||
| Clonal bone marrow plasma cells ≥ 60% | ||
| Involved: uninvolved serum free light chain ratio ≥ 100 (involved free light chain level must be ≥ 100 mg per L) | ||
| More than one focal lesion on MRI studies (≥ 5 mm size) | ||
| Solitary plasmacytoma | All criteria must be met: | |
| (1) Biopsy-proven solitary lesion of bone or soft tissue with evidence of clonal plasma cells | ||
| (2) Normal bone marrow with no evidence of clonal plasma cells | ||
| (3) Normal skeletal survey and MRI or CT of spine and pelvis except for the primary solitary lesion | ||
| (4) Absence of myeloma-related organ or tissue impairment* or CRAB criteria that can be attributed to a lymphoplasma cell proliferative disorder | ||
| Solitary plasmacytoma with minimal marrow involvement | All criteria must be met: | |
| (1) Biopsy-proven solitary lesion of bone or soft tissue with evidence of clonal plasma cells | ||
| (2) Clonal bone marrow plasma cells < 10% | ||
| (3) Normal skeletal survey and MRI or CT of spine and pelvis except for the primary solitary lesion | ||
| (4) Absence of myeloma-related organ or tissue impairment* or CRAB criteria that can be attributed to a lymphoplasma cell proliferative disorder | ||
| Plasma cell leukemia | Peripheral blood circulating clonal plasma cells > 2,000 per μL (2.0 × 109 per L) or 20% of leukocytes defines plasma cell leukemia | |
| Presence of serum monoclonal protein, bone marrow infiltration, or myeloma-related organ or tissue impairment* or CRAB criteria supports, but is not required for, diagnosis | ||
| Waldenström macroglobulinemia | Both criteria must be met: | |
| (1) IgM monoclonal gammopathy (IgM myeloma rare) | ||
| (2) Bone marrow lymphoplasmacytic infiltration ≥ 10% | ||
| Other possible findings: | ||
| (1) Lymphoplasmacytic infiltrate expresses typical immunophenotype | ||
| (2) Anemia, hepatosplenomegaly, and systemic symptoms typically present | ||
| Amyloid light chain (primary) amyloidosis | All criteria must be met: | |
| (1) Presence of an amyloid-related systemic syndrome (e.g., renal, liver, heart, gastrointestinal tract, or peripheral nerve involvement) that can be attributed to a plasma cell proliferative disorder | ||
| (2) Positive amyloid staining by Congo red in any tissue (e.g., fat aspirate, bone marrow, organ biopsy) or the presence of amyloid fibrils on electron microscopy | ||
| (3) Evidence that the amyloid is light chain related, established by direct examination of the amyloid using spectrometry-based proteomic analysis or immunoelectron microscopy | ||
| (4) Evidence of a monoclonal plasma cell proliferative disorder (e.g., presence of a serum or urinary monoclonal protein, abnormal serum free light chain ratio, or clonal bone marrow plasma cells) | ||
| POEMS syndrome (polyneuropathy, organomegaly, endocrinopathy, M protein, skin changes) | Both criteria must be met: | |
| (1) Polyneuropathy | ||
| (2) Monoclonal plasma cell proliferative disorder | ||
| Plus at least one major criterion: | ||
| (1) Osteosclerotic or mixed sclerotic/lytic lesion measuring ≥ 0.8 cm in the longest dimension | ||
| (2) Castleman disease (giant lymph node hyperplasia, angiofollicular lymph node hyperplasia) | ||
| (3) Elevated serum vascular endothelial growth factor levels at least three to four times the upper limit of normal | ||
| Plus at least one minor criterion: | ||
| (1) Organomegaly (splenomegaly, hepatomegaly, or lymphadenopathy) | ||
| (2) Extravascular volume overload (peripheral edema, ascites, or pleural effusion) | ||
| (3) Endocrinopathy (excluding diabetes mellitus or hypothyroidism) | ||
| (4) Skin changes (hyperpigmentation, hypertrichosis, acrocyanosis, plethora, hemangioma, telangiectasia) | ||
| (5) Papilledema | ||
| (6) Thrombocytosis or polycythemia | ||
Diagnosis
The classic definition of multiple myeloma required a clonal proliferation of plasma cells with evidence of end-organ damage.6,13,20 Typically, the CRAB criteria are present: calcium (hypercalcemia), renal impairment, anemia, and bone involvement (osteolytic lesions such as those shown in Figure 2 and eFigure A). Clinical judgment is used in discerning if these findings are attributable to the monoclonal proliferative disorder. In November 2014, the International Myeloma Working Group (IMWG) revised the diagnostic criteria for several reasons: imaging is now able to detect asymptomatic disease, biomarkers can better predict progression, and evidence has shown that treating asymptomatic disease is beneficial.26–28 These new criteria add myeloma-defining events, the presence of any one of which is sufficient for diagnosis when clonal plasma cell proliferation or plasmacytoma has been detected20 (Table 31,2,6,13,14,20 ).
In patients who have been diagnosed with SMM, myeloma-defining events are associated with a very high risk of progression of multiple myeloma (80% within two years vs. 10% per year for the first five years in the absence of these features).20 One implication of the new IMWG criteria is that patients being evaluated for SMM should undergo whole-body magnetic resonance imaging or positron emission tomography and computed tomography rather than a plain film skeletal survey because the latter is less sensitive for detecting bone lesions.29
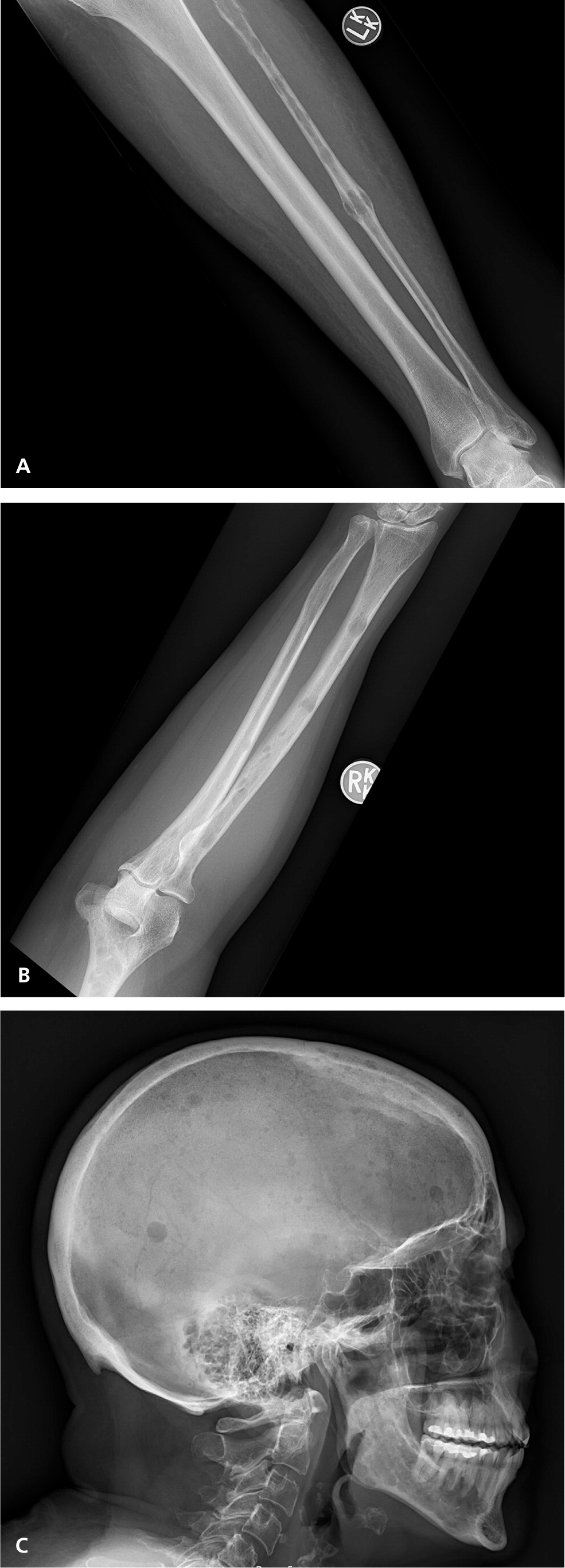
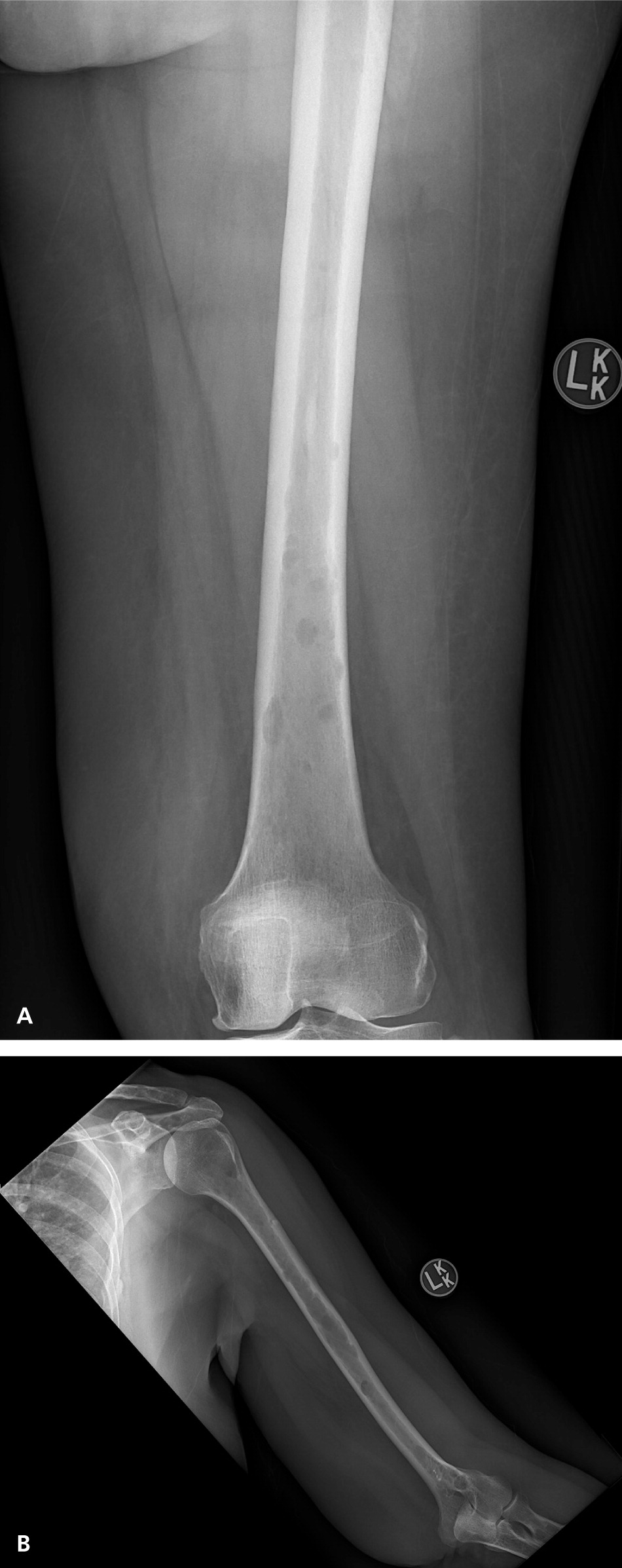
Staging and Prognosis
The International Staging System, which uses serum beta2-microglobulin and albumin levels, is the most widely adopted multiple myeloma staging system.30 Recently, the IMWG developed the Revised International Staging System, using traditional staging criteria plus the presence of chromosomal abnormalities by fluorescence in situ hybridization and serum lactate dehydrogenase levels.31 The newer staging system was found to predict progression-free and overall survival and has been recommended for use in future studies. Other guidelines recommend fluorescence in situ hybridization testing in all cases of newly diagnosed myeloma to identify high-risk patients.11,32 In the future, gene expression profiling may have a role in staging, but currently this procedure is neither validated nor practical.11,30,31 Table 4 outlines traditional and newer staging systems.1,2,31,32
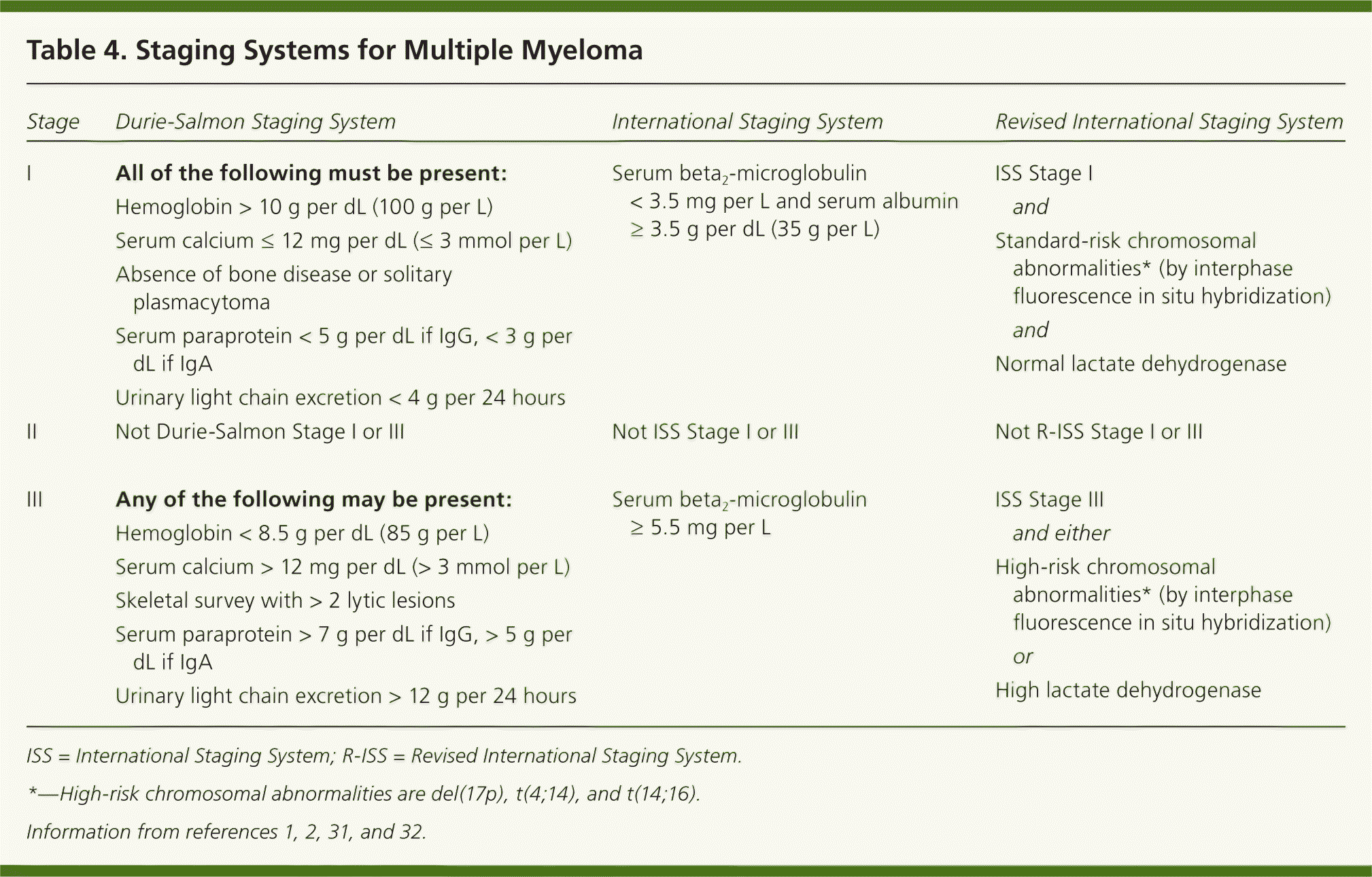
| Stage | Durie-Salmon Staging System | International Staging System | Revised International Staging System | ||
|---|---|---|---|---|---|
| I | All of the following must be present: | Serum beta2-microglobulin < 3.5 mg per L and serum albumin ≥ 3.5 g per dL (35 g per L) | ISS Stage I | ||
| Hemoglobin > 10 g per dL (100 g per L) | and | ||||
| Serum calcium ≤ 12 mg per dL (≤ 3 mmol per L) | Standard-risk chromosomal abnormalities* (by interphase fluorescence in situ hybridization) | ||||
| Absence of bone disease or solitary plasmacytoma | and | ||||
| Serum paraprotein < 5 g per dL if IgG, < 3 g per dL if IgA | Normal lactate dehydrogenase | ||||
| Urinary light chain excretion < 4 g per 24 hours | |||||
| II | Not Durie-Salmon Stage I or III | Not ISS Stage I or III | Not R-ISS Stage I or III | ||
| III | Any of the following may be present: | Serum beta2-microglobulin ≥ 5.5 mg per L | ISS Stage III | ||
| Hemoglobin < 8.5 g per dL (85 g per L) | and either | ||||
| Serum calcium > 12 mg per dL (> 3 mmol per L) | High-risk chromosomal abnormalities* (by interphase fluorescence in situ hybridization) | ||||
| Skeletal survey with > 2 lytic lesions | or | ||||
| Serum paraprotein > 7 g per dL if IgG, > 5 g per dL if IgA | High lactate dehydrogenase | ||||
| Urinary light chain excretion > 12 g per 24 hours | |||||
Treatment
Treatment for multiple myeloma has evolved, sequentially changing survival outlook. The development of proteasome inhibitors and immunomodulatory drugs has had the largest impact. Most recently, monoclonal antibodies have been approved for treatment of myeloma. Ancillary care for myeloma-related bone disease and other interventions have also resulted in improved survival. Median overall survival has increased, improving from a range of one to two years to seven to eight years, with a better quality of life and some patients achieving long-term survival. Five-year survival progressed from approximately 30% in 1990 to 45% in 2007.2,3,6,11,14
In deciding on treatment options, oncologists take into account disease-specific parameters, such as stage and gene expression profile of the clonal plasma cells, and patient factors, including comorbid illnesses and functional status. The optimal treatment for multiple myeloma is two- or three-drug myeloablative chemotherapy, followed by autologous stem cell transplantation (ASCT). ASCT improves median survival in patients with multiple myeloma by approximately 12 months, with a 10% long-term survival rate in some studies.13–15,33 Older patients and those with substantial comorbidities have typically not been candidates for this therapy and were offered alternative chemotherapy instead. However, more recent evidence indicates that age itself does not preclude benefit from this treatment, and healthy adults in their 70s are now offered this more aggressive management.33,34 Assessment of older patients as fit, frail, or intermediate has been shown to predict discontinuation of therapy and overall survival.35
Patients who are candidates for ASCT are typically treated with induction chemotherapy, followed by transplantation. After ASCT, short-term consolidation therapy is used, followed by maintenance therapy designed to prolong the response at minimal toxicity.11,12,14 Patients who are not candidates for ASCT are initially treated with chemotherapy.16 Based on good response rates to newer chemotherapeutic agents in patients not eligible for ASCT, some have questioned whether these treatments should replace transplantation; ongoing studies will clarify the answer to this question.6,15 Different chemotherapeutic drugs and their main adverse effects are presented in Table 5.1,2,13,15,16,36 The specific agents used at each stage of disease are beyond the scope of this article.
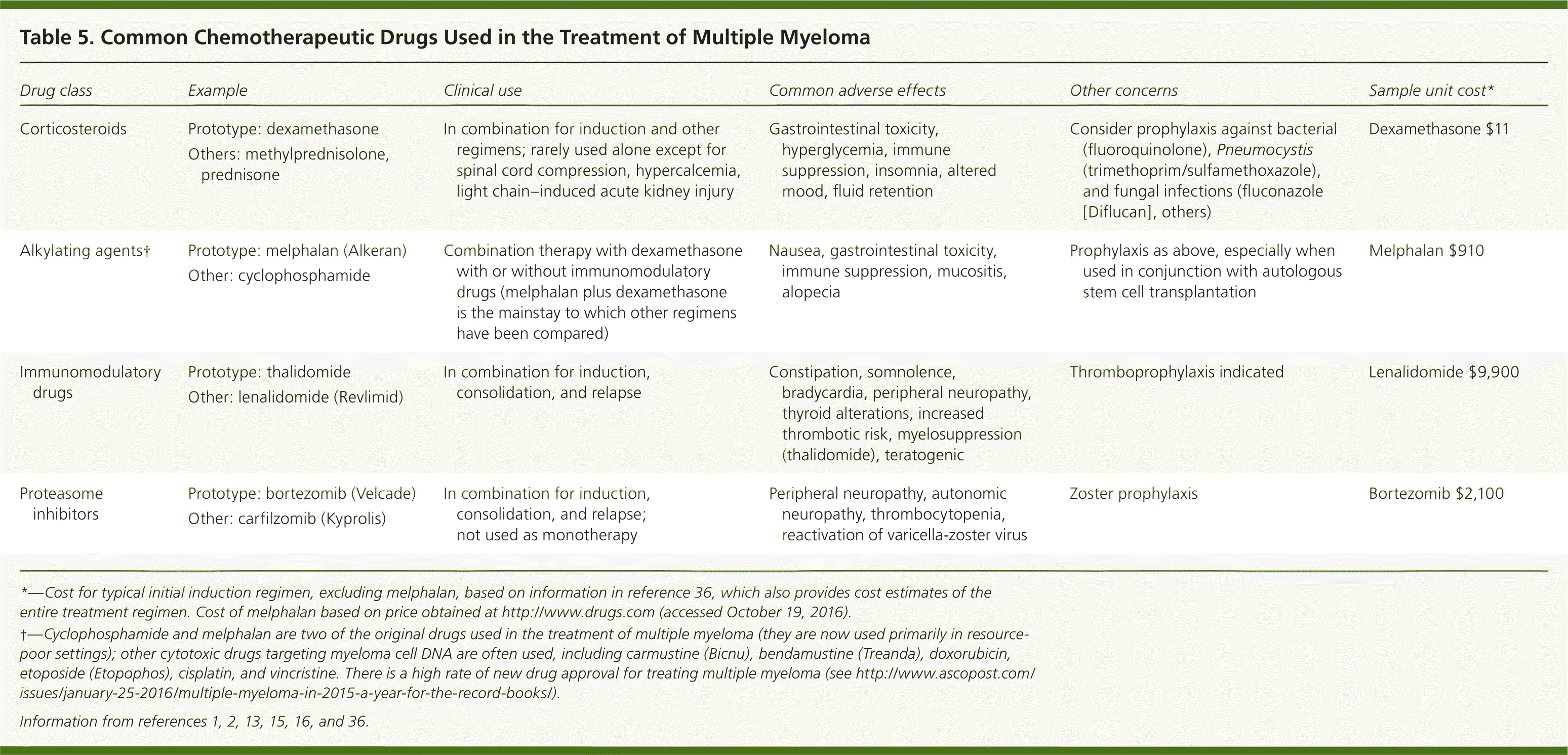
| Drug class | Example | Clinical use | Common adverse effects | Other concerns | Sample unit cost* |
|---|---|---|---|---|---|
| Corticosteroids |
| In combination for induction and other regimens; rarely used alone except for spinal cord compression, hypercalcemia, light chain–induced acute kidney injury | Gastrointestinal toxicity, hyperglycemia, immune suppression, insomnia, altered mood, fluid retention | Consider prophylaxis against bacterial (fluoroquinolone), Pneumocystis (trimethoprim/sulfamethoxazole), and fungal infections (fluconazole [Diflucan], others) | Dexamethasone $11 |
| Alkylating agents† |
| Combination therapy with dexamethasone with or without immunomodulatory drugs (melphalan plus dexamethasone is the mainstay to which other regimens have been compared) | Nausea, gastrointestinal toxicity, immune suppression, mucositis, alopecia | Prophylaxis as above, especially when used in conjunction with autologous stem cell transplantation | Melphalan $910 |
| Immunomodulatory drugs |
| In combination for induction, consolidation, and relapse | Constipation, somnolence, bradycardia, peripheral neuropathy, thyroid alterations, increased thrombotic risk, myelosuppression (thalidomide), teratogenic | Thromboprophylaxis indicated | Lenalidomide $9,900 |
| Proteasome inhibitors |
| In combination for induction, consolidation, and relapse; not used as monotherapy | Peripheral neuropathy, autonomic neuropathy, thrombocytopenia, reactivation of varicella-zoster virus | Zoster prophylaxis | Bortezomib $2,100 |
Select patients with SMM who are at increased risk of progression should be considered for treatment. These decisions involve discussion between the patient and oncologist; such patients should be considered for enrollment in ongoing clinical trials.12
Specific Treatment Issues
RENAL DISEASE
Impairment of renal function is common in patients with multiple myeloma and can occur for a number of reasons, including free light chain damage to the proximal tubules, hypercalcemia, hyperuricemia, volume depletion, infections, and adverse effects of nephrotoxic drugs. Renal toxic drugs and imaging studies with contrast media should be avoided in patients with myeloma. Patients presenting with an acute kidney injury need treatment directed at the underlying cause. Intravenous normal saline of at least 3 L per day is recommended. In patients with kidney injury and elevated serum light chains, dexamethasone is typically used with chemotherapy to reduce light chain load.11,12
BONE DISEASE
Bone disease develops in 80% to 90% of patients with myeloma and includes bone pain, pathologic fractures (40%), spinal cord compression (5%), and hypercalcemia. These skeletal-related events compromise mobility and independence, adversely affect quality of life, and are associated with decreased survival.2,37 The 2013 IMWG consensus statement on the treatment of bone disease recommends intravenous zoledronic acid (Reclast) or pamidronate for all patients with multiple myeloma who are receiving treatment, regardless of the presence of bone lesions.37 Both of these bisphosphonates have been shown to decrease vertebral compression fractures and other bone complications; zoledronic acid was shown to improve survival in one randomized controlled trial.37,38
Patients with multiple myeloma should take calcium and vitamin D3 supplements; calcium should be used cautiously in patients with renal insufficiency. For patients who have sustained a vertebral compression fracture, balloon kyphoplasty has been shown to improve pain and function. Patients with actual or impending spinal cord compression should receive orthopedic or neurosurgical consultation.12,37
THROMBOEMBOLIC EVENTS
Without prophylaxis, thromboembolic events are extremely common in patients with multiple myeloma, especially those receiving immunomodulatory drugs. Prospective randomized trials have shown a reduction in the incidence of thromboembolic complications from 12% to 26% to rates of 5% to 8% or less with low-molecular-weight heparin, warfarin (Coumadin), or aspirin.39,40 Patients treated for multiple myeloma, especially those receiving immunomodulatory drugs, should receive thromboprophylaxis for four to six months when first diagnosed or until their disease is controlled.41,42 The American Society of Clinical Oncology recommends using low-molecular-weight heparin or warfarin (target international normalized ratio = 2 to 3).39 The IMWG recommends stratification based on typical thromboembolic risk factors and that aspirin alone be considered for the lowest-risk patients.39,41
INFECTION
Patients with multiple myeloma are at high risk of serious infections; prompt recognition and treatment are critical. Prophylactic antibiotics are recommended in certain situations; this includes use of trimethoprim/sulfamethoxazole or a fluoroquinolone for the first three months of treatment.16 Prophylactic penicillin is prescribed only for recurrent pneumococcal infections; intravenous immune globulin is given for recurrent serious bacterial infections. Prophylactic antiviral treatment is recommended for patients taking proteasome inhibitors because of the risk of varicella-zoster virus reactivation.11,14 Immunization against pneumococcal pneumonia, Haemophilus influenzae, and influenza virus is recommended, especially for patients receiving ASCT; vaccinations should be administered at diagnosis, after evaluation of the patient's immune status, or at least two weeks before or several months after the transplantation.12,42,43
OTHER COMPLICATIONS
Patients with multiple myeloma often develop anemia. Evidence supports a restrictive transfusion policy (i.e., transfusion for hemoglobin levels less than 7 g per dL [70 g per L] in most patients); erythropoiesis-stimulating agents decrease the need for red blood cell transfusions, but increase the risk of thromboembolic complications and death.44 High concentrations of serum paraproteins (e.g., IgG 4 to 10 g per dL) cause hyperviscosity syndrome, which is treated with urgent plasma exchange and antimyeloma chemotherapy.11
Role of the Family Physician
Family physicians should have a high index of suspicion for multiple myeloma because presenting symptoms are often nonspecific. Patients with plasma cell disorders should be monitored for complications such as the development of bone pain, peripheral neuropathy, infections, thromboembolism, weight loss, fatigue, and treatment toxicity such as cytopenias and gastrointestinal symptoms. Patients with MGUS and SMM should also have regular monitoring of paraprotein and serum light chain levels. Addressing nutritional issues and pain control is an important concern. Pain management is multimodal with use of analgesic and neuropathic medications, radiation therapy, and psychological support. A regular exercise program has been shown to improve quality of life in patients with hematologic malignancies.45 End-of-life care is a strength of family physicians and includes managing symptoms and addressing unmet holistic needs and caregiver support.46
This article updates previous articles on this topic by George and Sadovsky47 and by Nau and Lewis.5
Data Sources: The authors searched for multiple myeloma in Essential Evidence Plus, the Cochrane Database of Systematic Reviews, National Guideline Clearinghouse, UpToDate, and DynaMed. Pubmed and Ovid searches were restricted to review articles. Specific journals, such as American Family Physician and New England Journal of Medicine, were searched for all articles on multiple myeloma published since 2010. Search dates: November 27, 2015; December 15, 2015; January 4, 2016; January 13, 2016; and May 10, 2016.
The authors would like to thank Ross Michels, MD, for his recommendations on revising the manuscript.
The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the U.S. Army Medical Department or the U.S. Army Service at large.
