Am Fam Physician. 2020;102(1):30-38
Author disclosure: Dr. Raval is involved in research in the field of therapeutic apheresis, in particular optimizing the use of therapeutic plasma exchange, red cell exchange, and extracorporeal photopheresis. He has received funding to serve as a medical advisor and speaker for Terumo BCT, Alexion, and Sanofi Genzyme on these topics. This manuscript addresses practical blood banking and transfusion medicine and is unrelated to the areas of therapeutic apheresis for which Dr. Raval has received funding; the other authors have no relevant financial affiliations.
Millions of units of blood products are transfused annually to patients in the United States. Red blood cells are transfused to improve oxygen-carrying capacity in patients with or at high risk of developing symptomatic anemia. Restrictive transfusion thresholds with lower hemoglobin levels are typically clinically equivalent to more liberal thresholds. Transfusion of plasma corrects clinically significant coagulopathy in patients with or at high risk of bleeding. Mildly abnormal laboratory coagulation values are not predictive of clinical bleeding and should not be corrected with plasma. Transfused platelets prevent or treat bleeding in patients with thrombocytopenia or platelet dysfunction. Cryoprecipitate is transfused to treat hypofibrinogenemia. Many adverse reactions can occur during or after blood product transfusion. Transfusion-associated circulatory overload (i.e., volume overload) is the most common cause of mortality associated with blood products. Modifications to blood products can prevent or decrease the risks of transfusion-related adverse reactions. It is critical to quickly recognize when a reaction is occurring, stop the transfusion, assess, and support the patient. Reporting a reaction to the blood bank is part of ensuring patient safety and supporting hemovigilance efforts.
The transfusion of blood products is a common medical procedure, with more than 16 million units transfused annually in the United States.1 However, there are associated hazards. When considering transfusion of any blood product, it is good practice to consider the patient's relevant laboratory data, overall clinical circumstances, feasible alternatives, and adjuncts to transfusion.2 These factors should be part of the informed consent process.
| Recommendation | Sponsoring organization |
|---|---|
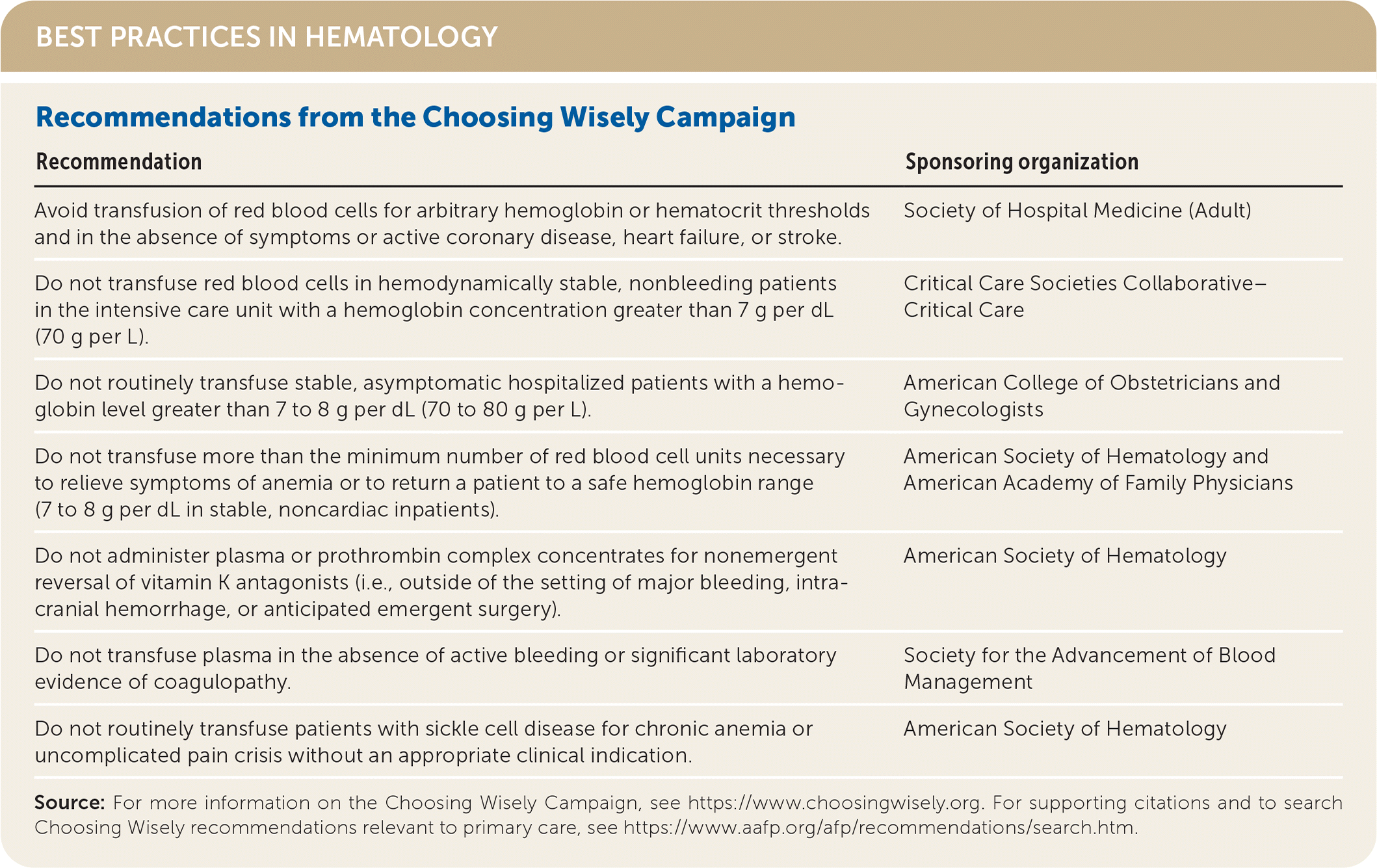
| Avoid transfusion of red blood cells for arbitrary hemoglobin or hematocrit thresholds and in the absence of symptoms or active coronary disease, heart failure, or stroke. | Society of Hospital Medicine (Adult) |
| Do not transfuse red blood cells in hemodynamically stable, nonbleeding patients in the intensive care unit with a hemoglobin concentration greater than 7 g per dL (70 g per L). | Critical Care Societies Collaborative–Critical Care |
| Do not routinely transfuse stable, asymptomatic hospitalized patients with a hemoglobin level greater than 7 to 8 g per dL (70 to 80 g per L). | American College of Obstetricians and Gynecologists |
| Do not transfuse more than the minimum number of red blood cell units necessary to relieve symptoms of anemia or to return a patient to a safe hemoglobin range (7 to 8 g per dL in stable, noncardiac inpatients). | American Society of Hematology and American Academy of Family Physicians |
| Do not administer plasma or prothrombin complex concentrates for nonemergent reversal of vitamin K antagonists (i.e., outside of the setting of major bleeding, intracranial hemorrhage, or anticipated emergent surgery). | American Society of Hematology |
| Do not transfuse plasma in the absence of active bleeding or significant laboratory evidence of coagulopathy. | Society for the Advancement of Blood Management |
| Do not routinely transfuse patients with sickle cell disease for chronic anemia or uncomplicated pain crisis without an appropriate clinical indication. | American Society of Hematology |
Indications
RED BLOOD CELLS
Red blood cells (RBCs) are transfused to increase oxygen-carrying capacity in patients with or at high risk of developing symptomatic anemia. In adults, RBCs are typically infused 1 unit at a time and increase hemoglobin by 1 g per dL (10 g per L). Guidelines for RBC transfusion from the AABB (formerly the American Association of Blood Banks) and others are provided in Table 1.2–9 For conditions that do not have specific guidelines, consider the patient's clinical situation and risk of adverse reactions for the transfusion threshold; however, most clinical trials demonstrate that patients do equally well with restrictive transfusion thresholds (hemoglobin less than 7 to 8 g per dL [70 to 80 g per L]) compared with liberal thresholds (hemoglobin less than 10 g per dL [100 g per L]).2,3,6
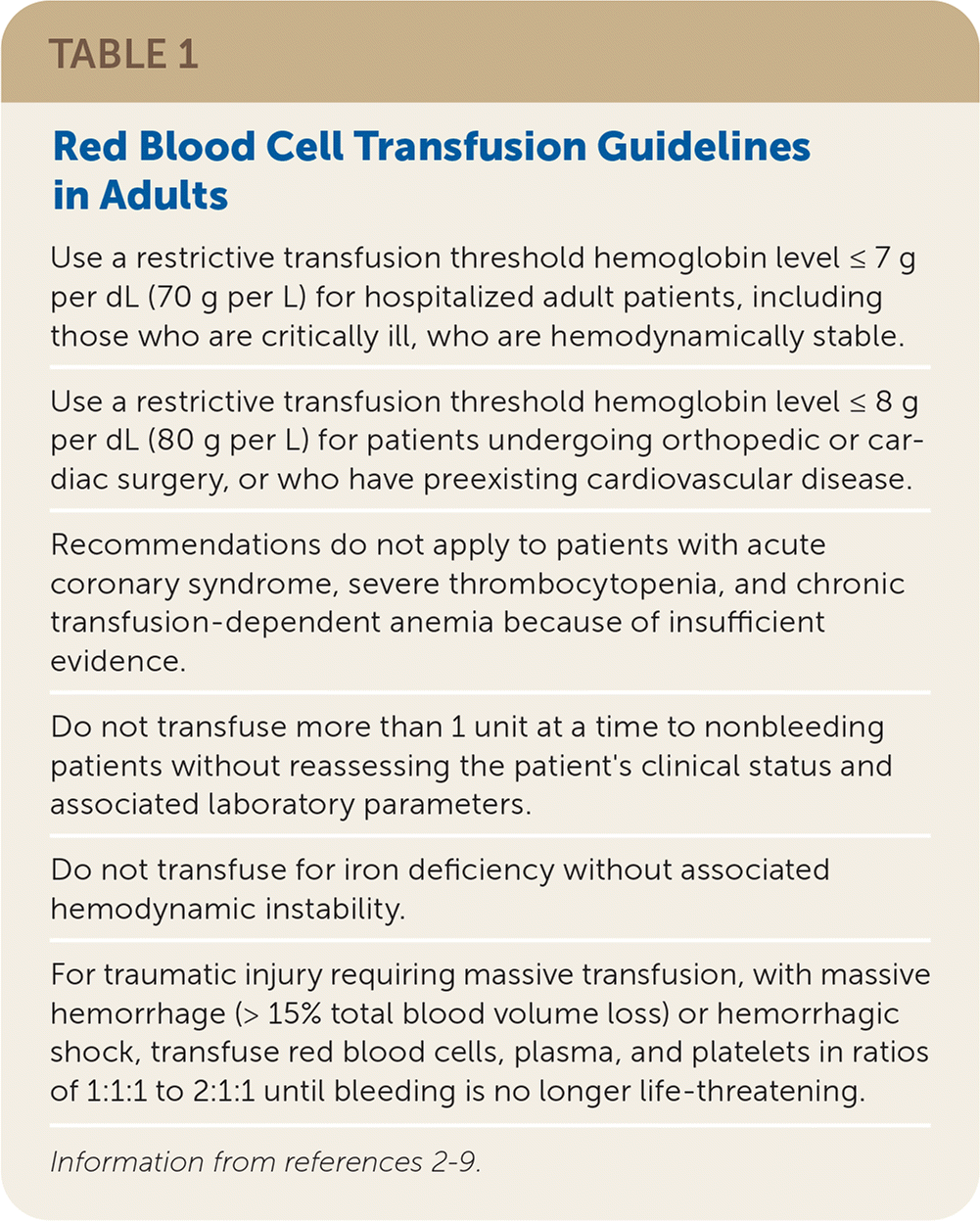
| Use a restrictive transfusion threshold hemoglobin level ≤ 7 g per dL (70 g per L) for hospitalized adult patients, including those who are critically ill, who are hemodynamically stable. |
| Use a restrictive transfusion threshold hemoglobin level ≤ 8 g per dL (80 g per L) for patients undergoing orthopedic or cardiac surgery, or who have preexisting cardiovascular disease. |
| Recommendations do not apply to patients with acute coronary syndrome, severe thrombocytopenia, and chronic transfusion-dependent anemia because of insufficient evidence. |
| Do not transfuse more than 1 unit at a time to nonbleeding patients without reassessing the patient's clinical status and associated laboratory parameters. |
| Do not transfuse for iron deficiency without associated hemodynamic instability. |
| For traumatic injury requiring massive transfusion, with massive hemorrhage (> 15% total blood volume loss) or hemorrhagic shock, transfuse red blood cells, plasma, and platelets in ratios of 1:1:1 to 2:1:1 until bleeding is no longer life-threatening. |
PLASMA
Plasma is transfused to correct a clinically significant coagulopathy in patients with or at high risk of bleeding. Typical plasma dosing is 10 to 20 mL per kg of body weight. Guidelines for plasma transfusion are provided in Table 2.4,6–12 The transfusion threshold of plasma for most adults should be an international normalized ratio greater than 1.5 to 1.6 in patients with active bleeding or at high risk of bleeding; however, some facilities set a higher threshold.4–8,11
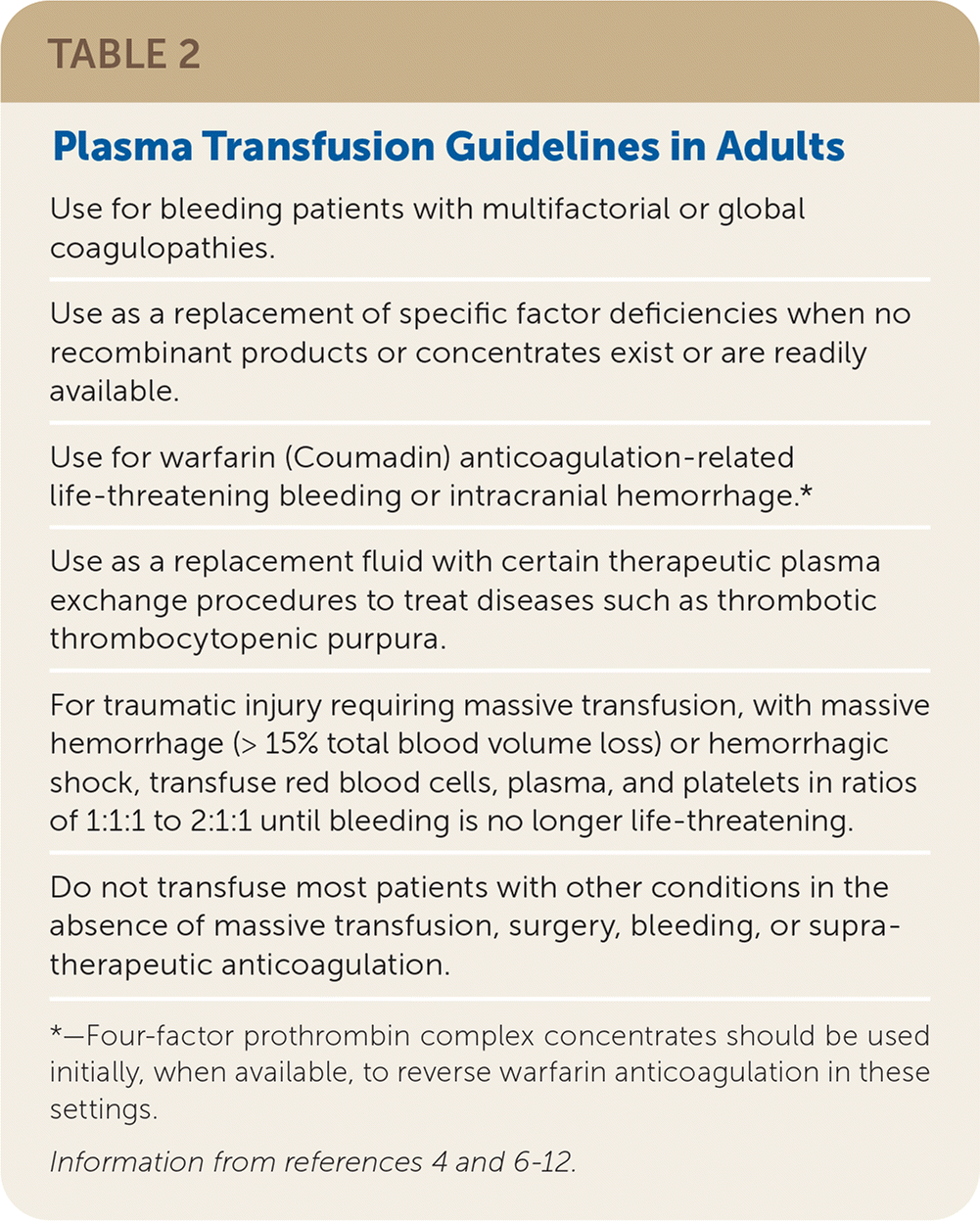
| Use for bleeding patients with multifactorial or global coagulopathies. |
| Use as a replacement of specific factor deficiencies when no recombinant products or concentrates exist or are readily available. |
| Use for warfarin (Coumadin) anticoagulation-related life-threatening bleeding or intracranial hemorrhage.* |
| Use as a replacement fluid with certain therapeutic plasma exchange procedures to treat diseases such as thrombotic thrombocytopenic purpura. |
| For traumatic injury requiring massive transfusion, with massive hemorrhage (> 15% total blood volume loss) or hemorrhagic shock, transfuse red blood cells, plasma, and platelets in ratios of 1:1:1 to 2:1:1 until bleeding is no longer life-threatening. |
| Do not transfuse most patients with other conditions in the absence of massive transfusion, surgery, bleeding, or supratherapeutic anticoagulation. |
Mildly abnormal laboratory coagulation tests poorly predict the occurrence of bleeding.6,12 A thorough bleeding history can help assess future bleeding risk and the need for additional coagulation testing.13 Prophylactic plasma transfusion in patients with coagulation abnormalities before surgical or interventional procedures does not nullify bleeding risk.6–8 The utility of prophylactic plasma transfusion in surgical patients without massive hemorrhage or for warfarin (Coumadin) anticoagulation reversal without intracranial hemorrhage is unclear because of the low quality of published evidence.11 Four-factor prothrombin complex concentrate is now the preferred product for warfarin anticoagulation reversal.7,8
PLATELETS
Platelets are transfused to prevent or treat bleeding associated with thrombocytopenia or platelet dysfunction. In adults, 1 unit of apheresis platelets increases platelet counts by 30,000 to 50,000 per μL (30 to 50 × 109 per L). Transfusion thresholds vary considerably based on the clinical situation.14–16
Guidelines for platelet transfusion in adults are provided in Table 3.4,6–9,14–18 The transfusion threshold of platelets for most adults before surgery or childbirth should be a platelet count of less than 50,000 per μL (50 × 109 per L).4,6–9,14–18 The utility of platelet transfusion for patients receiving antiplatelet therapy who have intracranial hemorrhage is unclear because of the low quality of published evidence.15

| Use in hospitalized adult patients with therapy-induced hypoproliferative thrombocytopenia and platelet count ≤ 10,000 per μL (10 × 109 per L). |
| Use for elective central venous catheter placement and platelet count < 20,000 per μL (20 × 109 per L). |
| Use for elective diagnostic lumbar puncture, major elective non-neuraxial surgery, or interventional procedures and platelet count < 50,000 per μL (50 × 109 per L). |
| Use for neuraxial surgery and platelet count < 100,000 per μL (100 × 109 per L). |
| For traumatic injury requiring massive transfusion, with massive hemorrhage (> 15% total blood volume loss) or hemorrhagic shock, transfuse red blood cells, plasma, and platelets in ratios of 1:1:1 to 2:1:1 until bleeding is no longer life-threatening. |
| Use for active bleeding in association with qualitative platelet defects regardless of platelet count. |
| Do not transfuse prophylactically to those undergoing cardiac surgery with cardiopulmonary bypass but not thrombocytopenia. |
CRYOPRECIPITATE
Cryoprecipitate contains coagulation factors VIII and XIII, fibrinogen, and von Willebrand factor. Initially used for factor VIII deficiency, cryoprecipitate is primarily transfused to correct acquired hy pofibrinogenemia. Transfusion thresholds typically are fibrinogen levels less than 100 to 150 mg per dL (1 to 1.5 g per L) but may vary depending on the clinical situation. One unit of cryoprecipitate (15 to 20 mL) per 5 to 7 kg of body weight increases fibrinogen concentration by up to 100 mg per dL.6–10
Adverse Reactions
If a reaction is suspected during transfusion, stop the infusion immediately and provide supportive care.6–8 Many findings associated with benign and life-threatening transfusion reactions are nonspecific and overlap; therefore, mild signs and symptoms should not be minimized. For any reaction, the implicated blood product bag, clinical reaction summary, and post-transfusion blood and urine samples from the patient should be sent to the blood bank to facilitate reaction investigation. If a reaction is suspected after the transfusion has ended, a reaction summary and post-transfusion blood and urine samples should be sent. See Table 4,6–9,19,20 Table 5,6–9,19,20 and Table 621 for information about noninfectious and infectious transfusion complications.
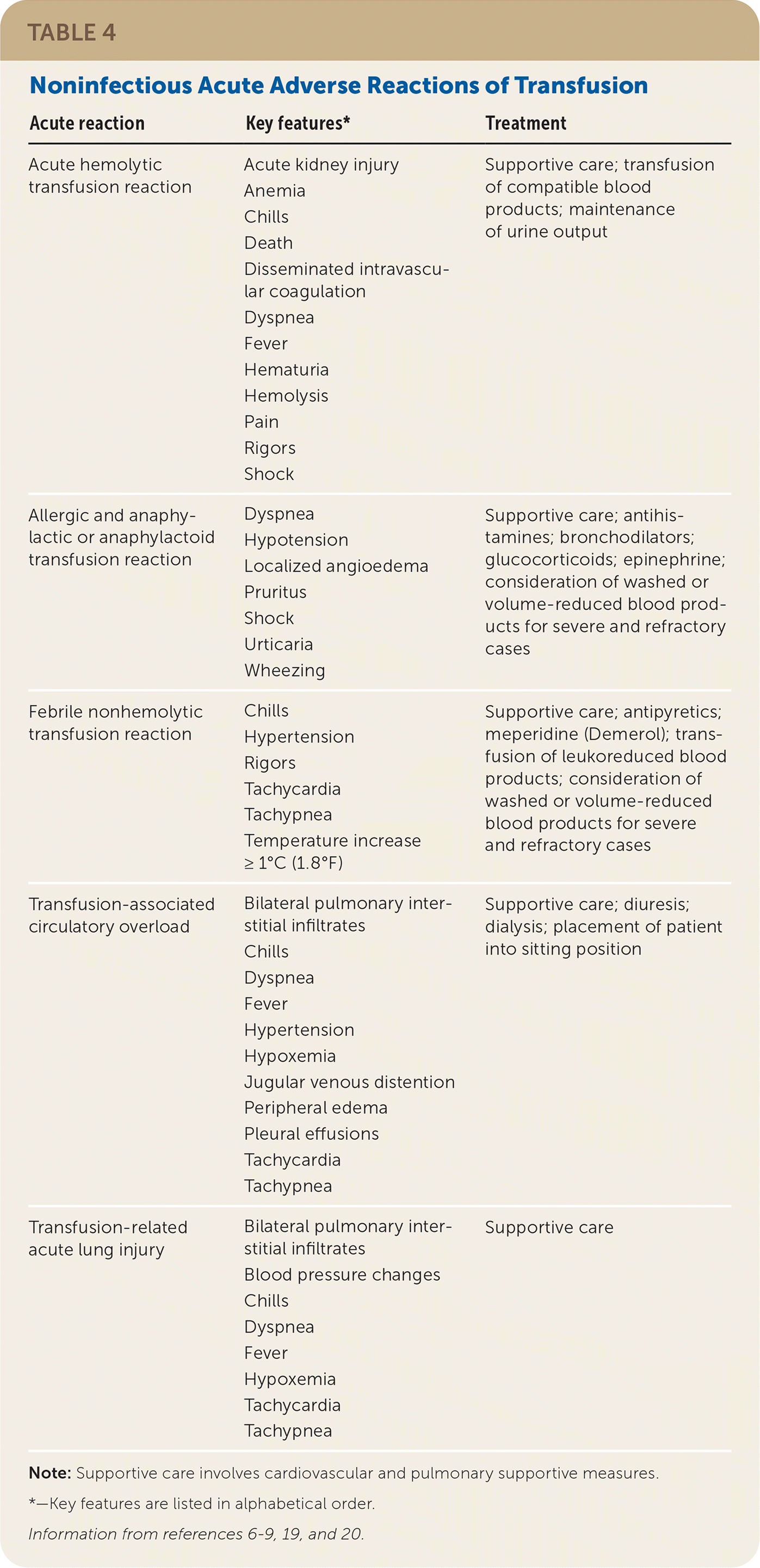
| Acute reaction | Key features* | Treatment |
|---|---|---|
| Acute hemolytic transfusion reaction | Acute kidney injury Anemia Chills Death Disseminated intravascular coagulation Dyspnea Fever Hematuria Hemolysis Pain Rigors Shock | Supportive care; transfusion of compatible blood products; maintenance of urine output |
| Allergic and anaphylactic or anaphylactoid transfusion reaction | Dyspnea Hypotension Localized angioedema Pruritus Shock Urticaria Wheezing | Supportive care; antihistamines; bronchodilators; glucocorticoids; epinephrine; consideration of washed or volume-reduced blood products for severe and refractory cases |
| Febrile nonhemolytic transfusion reaction | Chills Hypertension Rigors Tachycardia Tachypnea Temperature increase ≥ 1°C (1.8°F) | Supportive care; antipyretics; meperidine (Demerol); transfusion of leukoreduced blood products; consideration of washed or volume-reduced blood products for severe and refractory cases |
| Transfusion-associated circulatory overload | Bilateral pulmonary interstitial infiltrates Chills Dyspnea Fever Hypertension Hypoxemia Jugular venous distention Peripheral edema Pleural effusions Tachycardia Tachypnea | Supportive care; diuresis; dialysis; placement of patient into sitting position |
| Transfusion-related acute lung injury | Bilateral pulmonary interstitial infiltrates Blood pressure changes Chills Dyspnea Fever Hypoxemia Tachycardia Tachypnea | Supportive care |

| Delayed reaction | Key features* | Treatment |
|---|---|---|
| Alloimmunization | Difficulty obtaining compatible cellular blood products Hemolysis Occurs when antigen-negative patients are transfused with antigen-positive blood products Platelet refractoriness Production of antibodies against foreign antigens | Restrictive transfusion strategy; transfusion of compatible blood products |
| Delayed hemolytic transfusion reaction | Anemia Chills Dark urine Dyspnea Fever Jaundice Pain Rigors | Supportive care; transfusion of compatible blood products |
| Posttransfusion purpura | Bleeding Death Petechiae Purpura Rapid-onset thrombocytopenia | Supportive care; intravenous immunoglobulin; glucocorticoids; therapeutic plasma exchange |
| Transfusion-associated graft-versus-host disease | Abdominal pain Death Diarrhea Erythema Fever Hepatic dysfunction Maculopapular rash Nausea Pancytopenia Vomiting | Supportive care |
| Iron overload | Cardiomyopathy Endocrine organ dysfunction Hepatic injury | Restrictive transfusion strategy; iron chelation therapy |
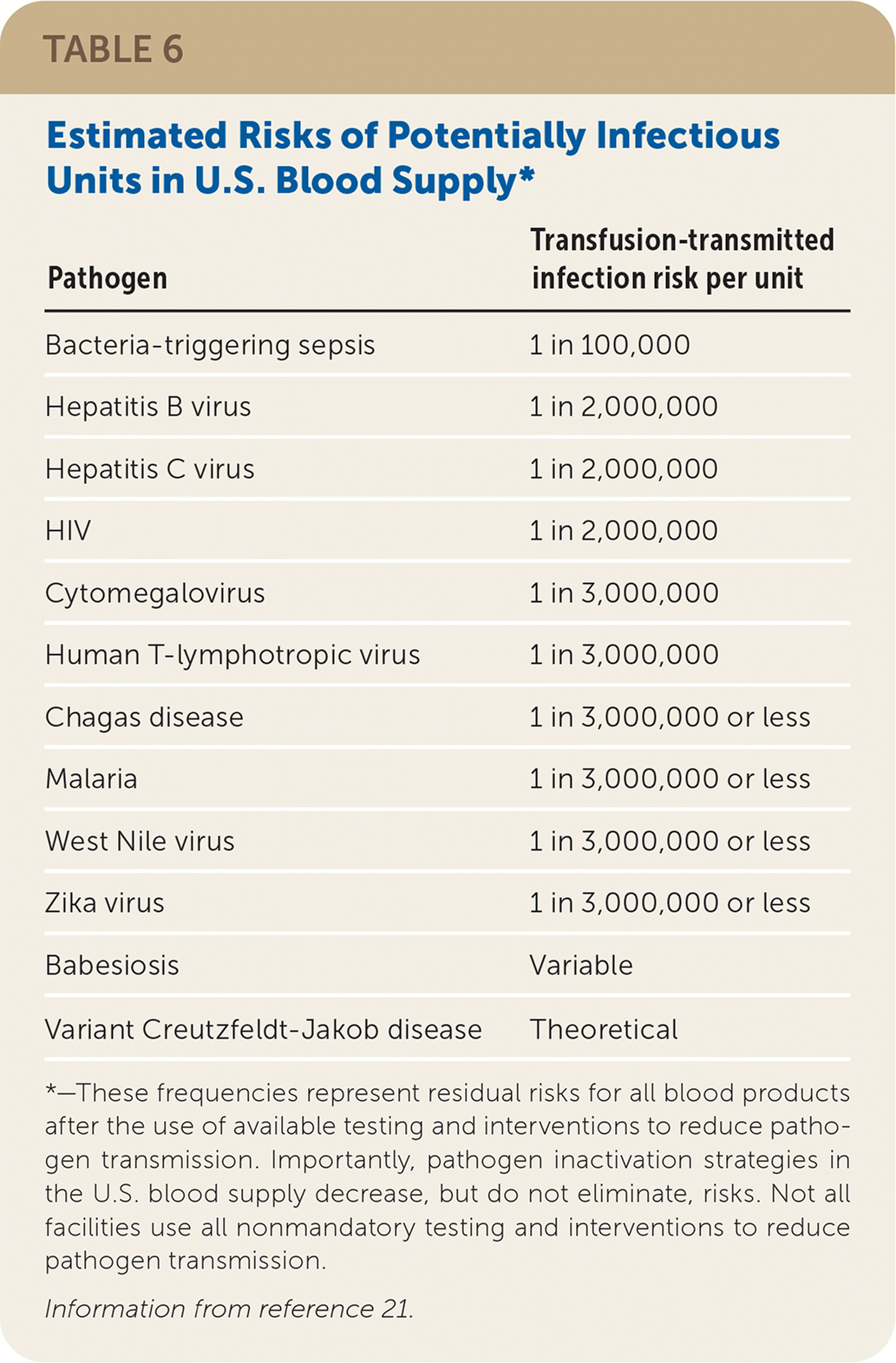
| Pathogen | Transfusion-transmitted infection risk per unit |
|---|---|
| Bacteria-triggering sepsis | 1 in 100,000 |
| Hepatitis B virus | 1 in 2,000,000 |
| Hepatitis C virus | 1 in 2,000,000 |
| HIV | 1 in 2,000,000 |
| Cytomegalovirus | 1 in 3,000,000 |
| Human T-lymphotropic virus | 1 in 3,000,000 |
| Chagas disease | 1 in 3,000,000 or less |
| Malaria | 1 in 3,000,000 or less |
| West Nile virus | 1 in 3,000,000 or less |
| Zika virus | 1 in 3,000,000 or less |
| Babesiosis | Variable |
| Variant Creutzfeldt-Jakob disease | Theoretical |
IMMUNE-MEDIATED REACTIONS
Febrile Nonhemolytic Transfusion Reaction (FNHTR). FNHTRs occur in 1% of transfusion episodes.7–9,19,20 They are characterized by a temperature increase of 1°C (1.8°F) or greater within four hours of transfusion and may be accompanied by chills, rigors, hypertension, tachycardia, and tachypnea. This is caused by recipient antibodies binding to white blood cells (WBCs) in the blood product, or proinflammatory cytokines produced within the product.19 This reaction is a diagnosis of exclusion, made only after other more serious reactions and any contributions from an underlying illness are ruled out. There should be no evidence of hemolysis or new bacterial infection. Treatment with antipyretics and meperidine (Demerol) can help control signs and symptoms. Universal pretransfusion use of anti-pyretics is not beneficial.22 However, their use in patients with a history of FNHTRs may allow for completion of transfusions.23 Prestorage leukoreduction of cellular blood products can help decrease the risk of FNHTRs (see Modifications). Washing and volume reduction also decrease FNHTRs with platelet transfusion.7–9,19
Allergic Transfusion Reaction. Allergic transfusion reactions occur with frequencies varying from 0.4% of RBC transfusions to 4.1% of platelet transfusions.7–9,14,19,20 Allergic transfusion reactions are mediated by immunoglobulin E (IgE) antibodies binding to allergens, ultimately resulting in the release of histamine. Allergic transfusion reactions range in severity from the most common form of isolated cutaneous involvement to localized angioedema and respiratory involvement to hypotension, shock, and complete cardiovascular collapse. Anaphylactic or anaphylactoid reactions, the most severe presentation of allergic transfusion reactions, occur in 8 per 100,000 units of blood products transfused.20 They typically occur during transfusion or within four hours after transfusion. Depending on reaction severity, antihistamines, glucocorticoids, bronchodilators, and epinephrine are all possible treatments.19,20 Volume reduction and washing decrease allergic transfusion reaction rates. Pretransfusion use of antihistamines or glucocorticoids has not been demonstrated to prevent the recurrence of mild allergic transfusion reactions despite widespread practice.19,20,22,23 In patients with severe allergic transfusion reactions, assess for IgA or haptoglobin deficiency.
Transfusion-Related Acute Lung Injury (TRALI). TRALI is defined as nonhydrostatic, noncardiogenic pulmonary edema occurring typically within six hours of transfusion.7–9,14,19,20,24 The current understanding of the pathophysiology is that antibody-dependent and -independent mechanisms result in neutrophil activation, endothelial injury, capillary leakage with exudative fluid extravasation, and ultimately acute lung injury. Findings include dyspnea, tachypnea, tachycardia, hypoxemia, fever, chills, blood pressure changes, and bilateral pulmonary interstitial infiltrates observed on chest radiography.24–27 Platelets have the highest risk of causing TRALI at 1 per 100,000 units, with RBCs and plasma at 0.5 and 0.4 per 100,000 units, respectively.14,28
Blood products donated by multiparous females pose a higher TRALI risk due to preformed WBC antibodies directed against foreign paternal antigens.29 Based on this role of WBC antibodies, risk reduction measures that screen or defer these higher-risk multiparous female donors have markedly decreased rates of TRALI, the previous leading cause of transfusion-associated mortality.30 Treatment consists of supportive care only because diuresis, steroids, and immunosuppressants are not helpful.25
Hemolytic Transfusion Reaction (HTR). An HTR is typically the antibody-mediated destruction of RBCs in a patient and can be attributed to incompatible RBCs or plasma.7–9,19 These reactions can be acute (occurring in less than 24 hours) or delayed (occurring at 24 hours or more), and hemolysis is classified as intravascular or extravascular. The most common cause of acute HTRs is human error.19,20 HTRs can be nonimmune in origin because of improper storage or administration, causing mechanical, thermal, or osmotic hemolysis.19,20 Hemolysis, fever, chills, jaundice, acute kidney injury, pain, shock, disseminated intravascular coagulation, and death are all possible complications. Supportive care is recommended for patients experiencing HTRs.
Transfusion-Associated Graft-Versus-Host Disease (TAGVHD). TAGVHD is a rare but almost universally fatal complication. It results from viable donor lymphocytes surviving, engrafting, and targeting recipient tissues.7–9,19,20 Patients at the highest risk of TAGVHD are typically severely immunocompromised (e.g., bone marrow transplant recipients, inherited deficiencies of cellular immunity, severe lymphopenia with absolute lymphocyte counts of less than 300 to 500 per μL [0.30 to 0.50 × 109 per L]). Classical findings including rash, fever, nausea, vomiting, diarrhea, pancytopenia, and liver injury can occur within five to 10 days of transfusion, with complete marrow aplasia within 21 days.19,20 Management is supportive only. Irradiation or pathogen inactivation can prevent TAGVHD (see Modifications).
NONIMMUNE-MEDIATED REACTIONS
Transfusion-Associated Circulatory Overload. Transfusion-associated circulatory overload occurs when the blood product infusion volume leads to iatrogenic cardiogenic pulmonary edema.7–9,19,24 Blood product volumes range from 150 mL for a dose of cryoprecipitate to 300 mL for a single RBC unit or platelets to liters for a weight-appropriate dose of plasma. Unlike with other fluids, little volume from blood products enters the third space, making them much riskier to infuse from an intravascular volume perspective. Rates of transfusion-associated circulatory overload range from 1% to 8%19,31,32; diminished heart and kidney function, compensated anemia, preexisting positive-fluid balance, cancer diagnosis, plasma ordered for reversing anticoagulation, and extremes of age are all parameters associated with increased transfusion-associated circulatory overload risk.
Findings in transfusion-associated circulatory overload are clinically similar to TRALI: new-onset or worsening dyspnea, tachypnea, tachycardia, hypoxemia, fever, chills, hypertension, and bilateral pulmonary interstitial infiltrates observed on chest radiography.19,24,31 Findings that differ from TRALI include jugular venous distention, peripheral edema, and elevated N-terminal pro-brain natriuretic peptide level.33 The most important aspect of managing transfusion-associated circulatory overload is to recognize it. Passive reporting of transfusion-associated circulatory overload underestimates its incidence.31,32 Prompt action with supportive care and diuresis or dialysis is required.
Transfusion-associated circulatory overload is the leading cause of transfusion-associated mortality.30 Strategies to reduce transfusion-associated circulatory overload in patients at high risk include slow transfusion over four hours and volume reduction (see Modifications). Although prophylactic peritransfusion diuresis has not been shown to reduce transfusion-associated circulatory overload,20 diuresis as part of an overall strategy for optimizing volume status may be beneficial.
Septic Reaction. Sepsis from a transfusion is attributed to bacterial growth in a blood product and occurs in 1 per 100,000 units transfused.20,21 Platelets have the highest rates of bacterial contamination (up to 1 in 3,000 units), most commonly with normal skin flora such as Staphylococcus and Streptococcus, because of their unique storage requirements.14,20 Symptoms typically occur within 24 hours of transfusion. To confidently diagnose a septic transfusion reaction, the patient and implicated blood product should have the same microorganism isolated34; however, a presumed reaction can be diagnosed if growth occurs only in the transfused product. Prompt recognition and rapid initiation of empiric antimicrobial therapy are essential. Broad-spectrum antibiotics are recommended, as are agents that can treat Pseudomonas and other gram-negative organisms if an RBC unit is implicated.34
Transfusion-Transmitted Infection. Aside from bacterial contamination, blood-borne infectious organisms can be transmitted by transfusion. Volunteer donors have their histories queried and limited physical examinations performed to assess for increased transfusion-transmitted infection risk. Patients who continue on to donation have their blood tested for a variety of infectious agents. Despite blood products being safer today than in the past, there are small but real risks of transfusion-transmitted infections (Table 6).21
Modifications
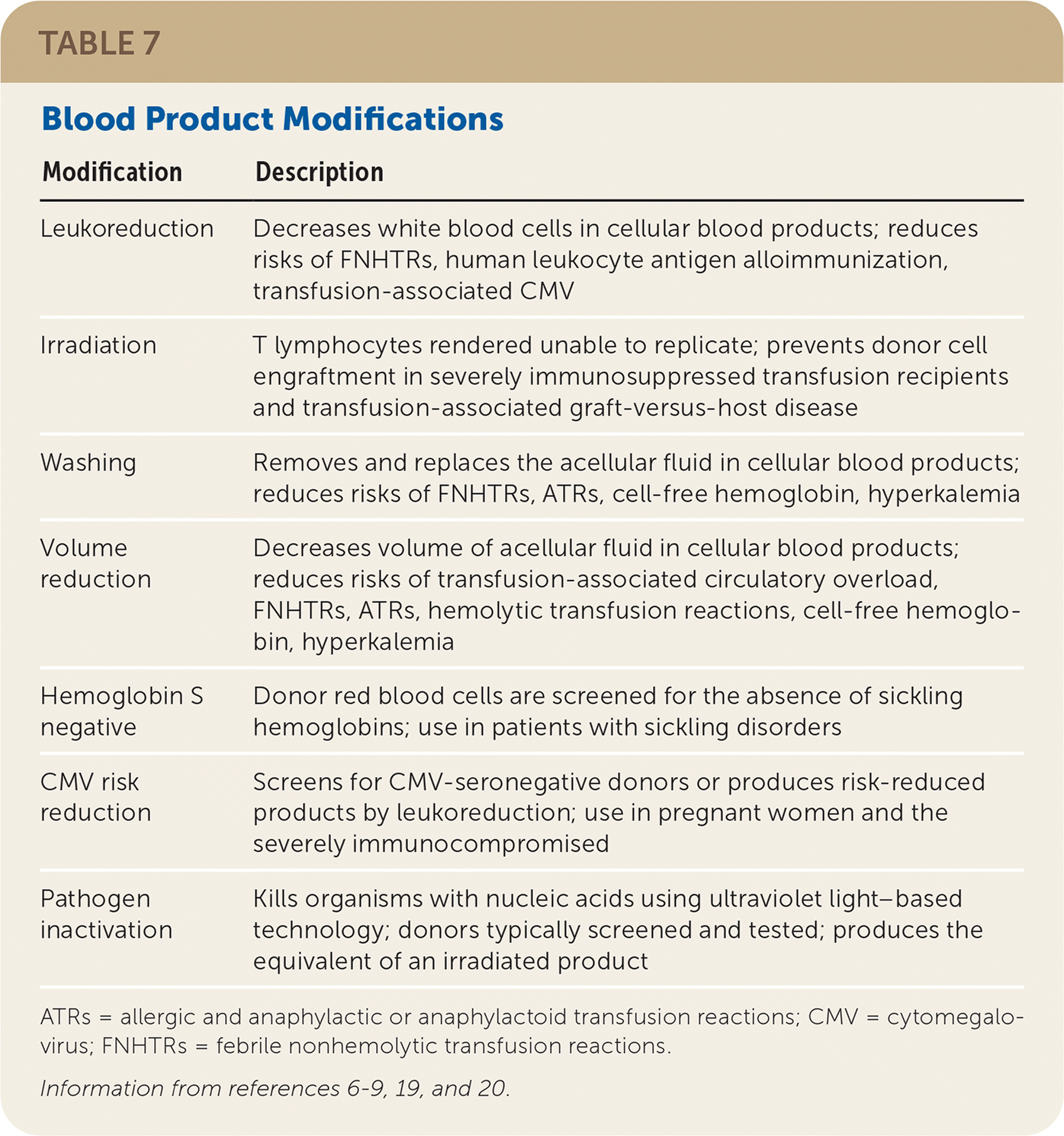
| Modification | Description |
|---|---|
| Leukoreduction | Decreases white blood cells in cellular blood products; reduces risks of FNHTRs, human leukocyte antigen alloimmunization, transfusion-associated CMV |
| Irradiation | T lymphocytes rendered unable to replicate; prevents donor cell engraftment in severely immunosuppressed transfusion recipients and transfusion-associated graft-versus-host disease |
| Washing | Removes and replaces the acellular fluid in cellular blood products; reduces risks of FNHTRs, ATRs, cell-free hemoglobin, hyperkalemia |
| Volume reduction | Decreases volume of acellular fluid in cellular blood products; reduces risks of transfusion-associated circulatory overload, FNHTRs, ATRs, hemolytic transfusion reactions, cell-free hemoglobin, hyperkalemia |
| Hemoglobin S negative | Donor red blood cells are screened for the absence of sickling hemoglobins; use in patients with sickling disorders |
| CMV risk reduction | Screens for CMV-seronegative donors or produces risk-reduced products by leukoreduction; use in pregnant women and the severely immunocompromised |
| Pathogen inactivation | Kills organisms with nucleic acids using ultraviolet light–based technology; donors typically screened and tested; produces the equivalent of an irradiated product |
LEUKOREDUCTION
Leukoreduction is WBC removal from RBCs or platelets. This process cannot eliminate all WBCs.7–9 Leukoreduction decreases the risk of three different complications: FNHTRs, transfusion-associated cytomegalovirus (CMV), and human leukocyte antigen alloimmunization. Most cellular blood products in the United States are leukoreduced before they are stored.
IRRADIATION
Irradiation of cellular blood products is performed to avoid TAGVHD in susceptible patients.7–9 This process prevents WBCs from replicating, which prevents TAGVHD. Irradiated cellular blood products should be transfused to patients with hematologic malignancies, bone marrow transplantation, inherited immune system disorders, and other conditions and situations that make patients susceptible to TAGVHD. Conditions such as HIV, sepsis, solid metastatic tumors, and solid organ transplantation do not require irradiated blood products.
WASHING
Washing removes cell-free fluid from cellular blood products and replaces it with an alternative fluid, which decreases the risk profile of these products.7–9,19 Patients with a history of documented severe allergic transfusion reactions, a diagnosis of IgA deficiency, or sensitivity to extracellular hemoglobin or potassium may benefit. Washing is a time- and labor-intensive manipulation that produces a quantitatively and qualitatively inferior product because RBCs are hemolyzed, and platelets are activated and lost. It may also decrease blood product shelf-life.
VOLUME REDUCTION
In patients who are at risk of developing transfusion-associated circulatory overload or who need to have offending substances removed from cell-free fluid in cellular blood products (similar to those requiring washed products), volume reduction can be considered.7–9,19 By decreasing cell-free fluid volume containing plasma and storage solution, the overall blood product volume is reduced; however, volume reduction is time- and labor-intensive and may also decrease blood product shelf-life.
HEMOGLOBIN S NEGATIVE
Patients with hemoglobin S disease or other sickle cell diseases cannot donate blood; however, patients with only the sickle cell trait can. Although RBCs from these donors are suitable for most patients, a patient with a sickle cell disease should not receive these units.35
CMV RISK REDUCTION
CMV is an intracellular virus that can cause complications in specific patient populations if transfused, including pregnant women (and their fetuses), bone marrow transplant recipients, patients with HIV, and other immunocompromised populations.7–9 For these patients, CMV risk-reduced blood products are recommended. Leukoreduction produces products with a decreased CMV risk profile comparable to that of blood products from CMV-seronegative donors.7–9,36
PATHOGEN INACTIVATION
Pathogen inactivation is a process through which the nucleic acid–containing elements within a blood product are intentionally damaged, producing pathogen-reduced blood product.7–9 This inactivation does not eliminate the need for routine processes surrounding blood donation. The pathogen inactivation process also injures the nucleic acids in WBCs and produces the equivalent of an irradiated blood product. Currently, pathogen inactivation technology can only be applied to plasma and platelets.
This article updates a previous article on this topic by Sharma, et al.37
Data Sources: PubMed and Cochrane Library searches were performed using the key terms transfusion, blood, indication, reaction, treatment, prevention, manipulation, and modification. The searches were restricted to human studies and included meta-analyses, randomized controlled trials, clinical trials, and reviews. Also searched were Essential Evidence Plus, critical reference texts in Transfusion Medicine, and relevant information from the U.S. Food and Drug Administration, Centers for Disease Control and Prevention, and AABB. Search dates: May 1, 2019, and March 1, 2020.