
A more recent article on outpatient management of COVID-19 is available.
Editor's Note: On February 27, 2021, the FDA issued an Emergency Use Authorization for the Janssen single-dose vaccine for the prevention of COVID-19 in individuals 18 years of age and older. Information on this vaccine's effectiveness and mechanism of action is available from the FDA and the Centers for Disease Control and Prevention. A summary of the FDA report can be found in AFP's COVID-19 Research Brief posted on March 1.
Am Fam Physician. 2020;102(8):478-486
Related editorial: Long COVID: A Primer for Family Physicians
Published online February 26, 2021.
Author disclosure: No relevant financial affiliations.
Common presenting symptoms of coronavirus disease 2019 include fever, dry cough, shortness of breath, and fatigue. However, patients may have a wide range of symptoms representing a spectrum of mild to severe illness. Symptoms in children tend to be milder and may include fever, cough, and feeding difficulty. The incubation period is two to 14 days, although symptoms typically appear within five days of exposure. Multiple testing modalities exist, but infection should be confirmed by polymerase chain reaction testing using a nasopharyngeal swab. Two vaccines are now available in the United States. There are no evidence-based treatments appropriate for use in the outpatient setting; management is supportive and should include education about isolation. In hospitalized patients, remdesivir should be considered to reduce time to recovery, and low-dose dexamethasone should be considered in patients who require supplemental oxygen. Overall, 85% of patients have mild illness, whereas 14% have severe disease requiring hospitalization, including 5% who require admission to an intensive care unit. Predictors of severe disease include increasing age, comorbidities, lymphopenia, neutrophilia, leukocytosis, low oxygen saturation, and increased levels of C-reactive protein, d-dimer, transaminases, and lactate dehydrogenase.
The coronavirus disease 2019 (COVID-19) pandemic is caused by an enveloped single-stranded RNA novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The first cases were reported in Wuhan City, China, in December 2019; the United States confirmed its first cases one month later. Middle East Respiratory Syndrome and Severe Acute Respiratory Syndrome, also caused by coronaviruses, have caused significantly lower global mortality.
Epidemiology
The basic reproductive number (infections per index case) of COVID-19 is unknown. One systematic review estimated a range of 1.9 to 6.5.1
Studies in the United States and Switzerland based on antibody sampling estimated seroprevalence between 1.1% and 10.9%.2
Pathogenic factors contributing to the virulence of SARS-CoV-2 include transmissibility via respiratory droplets and asymptomatic spread via healthy-appearing individuals.
The true incidence of COVID-19 in the United States is unknown and varies geographically.3
It is estimated that only one in approximately 80 COVID-19 infections was diagnosed in March 2020, and there is promise in disease modeling that extrapolates from influenza-like illness.4
Physical distancing of at least 6 ft (1.8 m) slows the spread of infection by decreasing the mean number of people infected per case, particularly when combined with other measures such as mask wearing in public, school closures, and travel restrictions.5,6
Contact tracing has reduced the spread of infection early during outbreaks in some countries.7
The Centers for Disease Control and Prevention (CDC) recommends that close contacts of a person with COVID-19 quarantine for 14 days after the last exposure and monitor for fever and other symptoms daily.8 People suspected of having COVID-19 should isolate within their home to prevent infection spread, stay in a specific “sick room” if they live with others, and use a separate bathroom, if possible.8
The CDC recommends wearing face masks when in a public space where appropriate physical distancing may be difficult.9 This practice may reduce viral transmission (Howard J, et al., and Kai D, et al., unpublished data, 2020), particularly from asymptomatic or presymptomatic individuals.10
Patients with respiratory symptoms should wear a surgical mask in health care settings. Those without respiratory symptoms should wear a cloth face mask per CDC recommendations.
Table 1 lists recommended personal protective equipment for clinicians based on different clinical scenarios.11–14
Two vaccines against SARS-CoV-2 are currently available in the United States: BNT162b2 (Pfizer/BioNTech) and mRNA-1273 (Moderna; Table 215,16). Both vaccines contain lipid-encapsulated mRNA molecules that are delivered to macrophages. Translation of mRNA results in expression of prefusion-stabilized spike proteins on the cellular surface and an immune reaction. The mRNA is subsequently degraded, and no genetic material enters the nucleus of cells.
Both vaccines were highly effective at preventing symptomatic infection in the short term in randomized, double-blind trials submitted to the U.S. Food and Drug Administration for Emergency Use Authorization. The mRNA-1273 vaccine trial included 30,420 patients 18 years and older, and the BNT162b2 mRNA vaccine trial included 43,548 participants 16 years and older.15,16
Currently, the data are insufficient to recommend vaccination of children and pregnant patients. We do not know whether the vaccine protects against asymptomatic infection or transmission.
The CDC and National Institutes of Health convened the National Academies of Sciences, Engineering, and Medicine to develop an overarching vaccine allocation framework.17 This framework is incorporated into the Advisory Committee on Immunization Practices COVID-19 vaccine recommendations, which support allocation of COVID-19 vaccines based on phases 1a, 1b, 1c, and 2 depending on factors such as age, occupation, and comorbidities.18 Updates are published in Morbidity and Mortality Weekly Report.19
More than 100 vaccines are in various stages of development. Vaccine strategies include inactivated viruses, replication-incompetent vectors, recombinant spike-proteins, and adenovirus-based DNA vaccines such as AZD1222 and JNJ-78436735.20 The AZD1222 (AstraZeneca) vaccine was approved for emergency use in the United Kingdom on December 30, 2020. Studies of the JNJ-78436735 (Johnson & Johnson) vaccine have been promising. Unlike the mRNA vaccines, some candidates can be stored at refrigerator temperatures or may be effective after one dose.
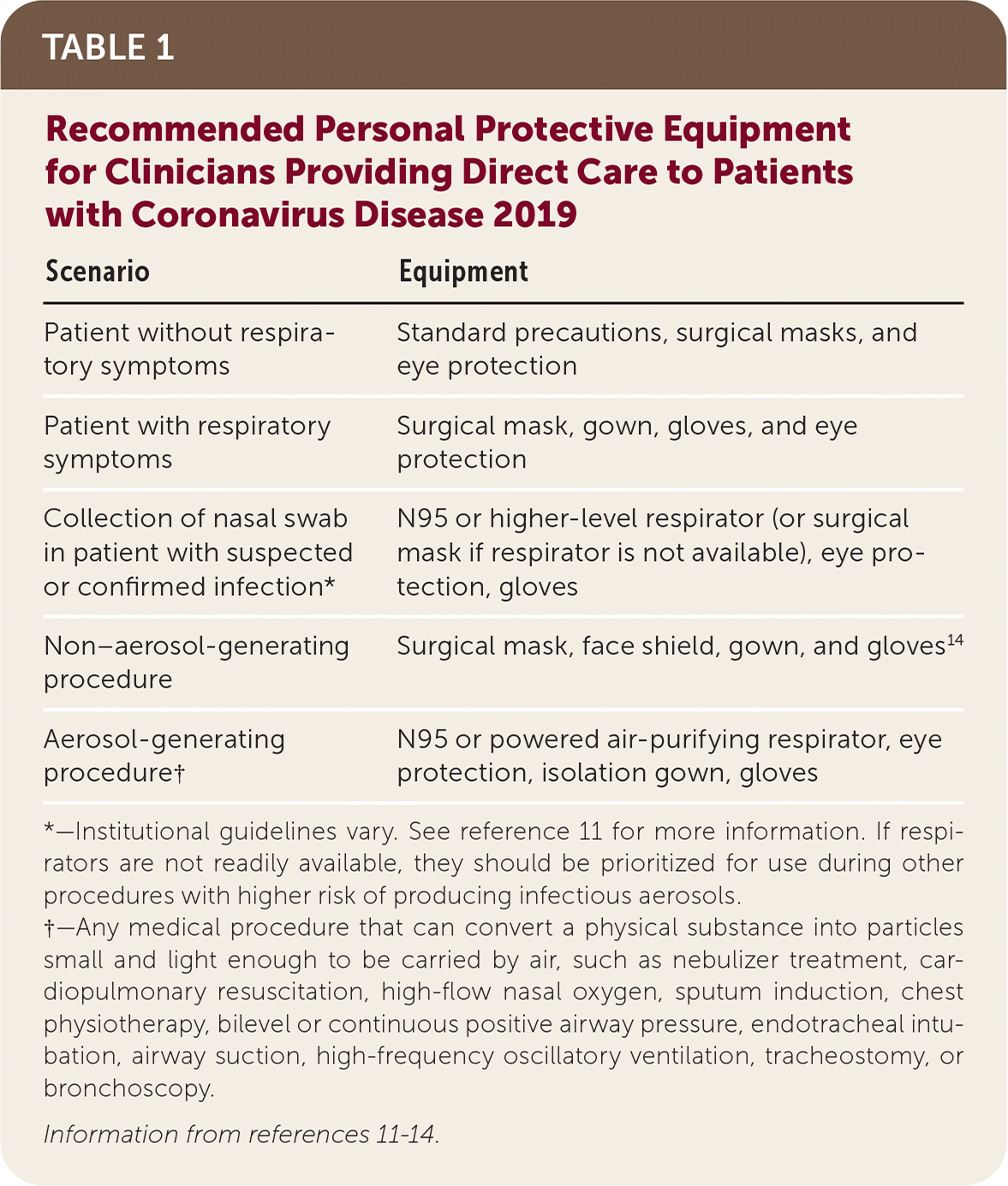
| Scenario | Equipment |
|---|---|
| Patient without respiratory symptoms | Standard precautions, surgical masks, and eye protection |
| Patient with respiratory symptoms | Surgical mask, gown, gloves, and eye protection |
| Collection of nasal swab in patient with suspected or confirmed infection* | Surgical mask, gown, gloves, and eye protection |
| Non–aerosol-generating procedure | Surgical mask, gown, gloves, and eye protection |
| Aerosol-generating procedure† | N95 or powered air-purifying respirator, eye protection, isolation gown, gloves |
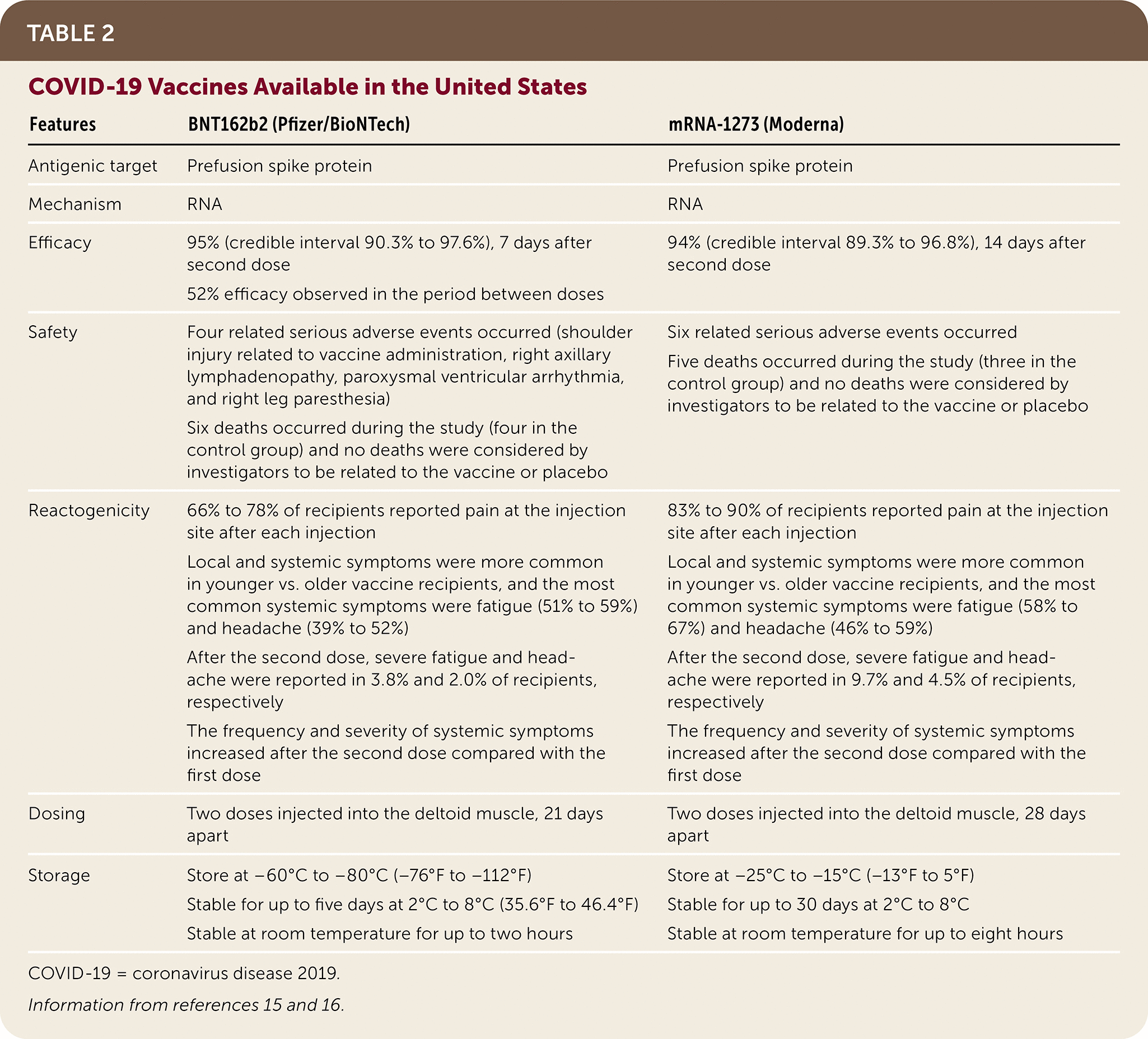
| Features | BNT162b2 (Pfizer/BioNTech | mRNA-1273 (Moderna) |
|---|---|---|
| Antigenic target | Prefusion spike protein | Prefusion spike protein |
| Mechanism | RNA | RNA |
| Efficacy | 95% (credible interval 90.3% to 97.6%), 7 days after second dose 52% efficacy observed in the period between doses | 94% (credible interval 89.3% to 96.8%), 14 days after second dose |
| Safety | Four related serious adverse events occurred (shoulder injury related to vaccine administration, right axillary lymphadenopathy, paroxysmal ventricular arrhythmia, and right leg paresthesia) Six deaths occurred during the study (four in the control group), and no deaths were considered by investigators to be related to the vaccine or placebo | Six related serious adverse events occurred Five deaths occurred during the study (three in the control group), and no deaths were considered by investigators to be related to the vaccine or placebo |
| Reactogenicity | 66% to 78% of recipients reported pain at the injection site after each injection Local and systemic symptoms were more common in younger vs. older vaccine recipients, and the most common systemic symptoms were fatigue (51% to 59%) and headache (39% to 52%) After the second dose, severe fatigue and headache were reported in 3.8% and 2.0% of recipients, respectively The frequency and severity of systemic symptoms increased after the second dose compared with the first dose | 83% to 90% of recipients reported pain at the injection site after each injection Local and systemic symptoms were more common in younger vs. older vaccine recipients, and the most common systemic symptoms were fatigue (58% to 67%) and headache (46% to 59%) After the second dose, severe fatigue and headache were reported in 9.7% and 4.5% of recipients, respectively The frequency and severity of systemic symptoms increased after the second dose compared with the first dose |
| Dosing | Two doses injected into the deltoid muscle, 21 days apart | Two doses injected into the deltoid muscle, 28 days apart |
| Storage | Store at –60°C to –80°C (–76°F to –112°F) Stable for up to five days at 2°C to 8°C (35.6°F to 46.4°F) Stable at room temperature for up to two hours | Store at –25°C to –15°C (–13°F to 5°F) Stable for up to 30 days at 2°C to 8°C Stable at room temperature for up to eight hours |
Diagnosis
The diagnosis of COVID-19 is made clinically and is supported by laboratory results and radiographic findings. COVID-19 should be suspected in patients with known or suspected exposure within the past 14 days, or in the setting of active SARS-CoV-2 transmission in the community.
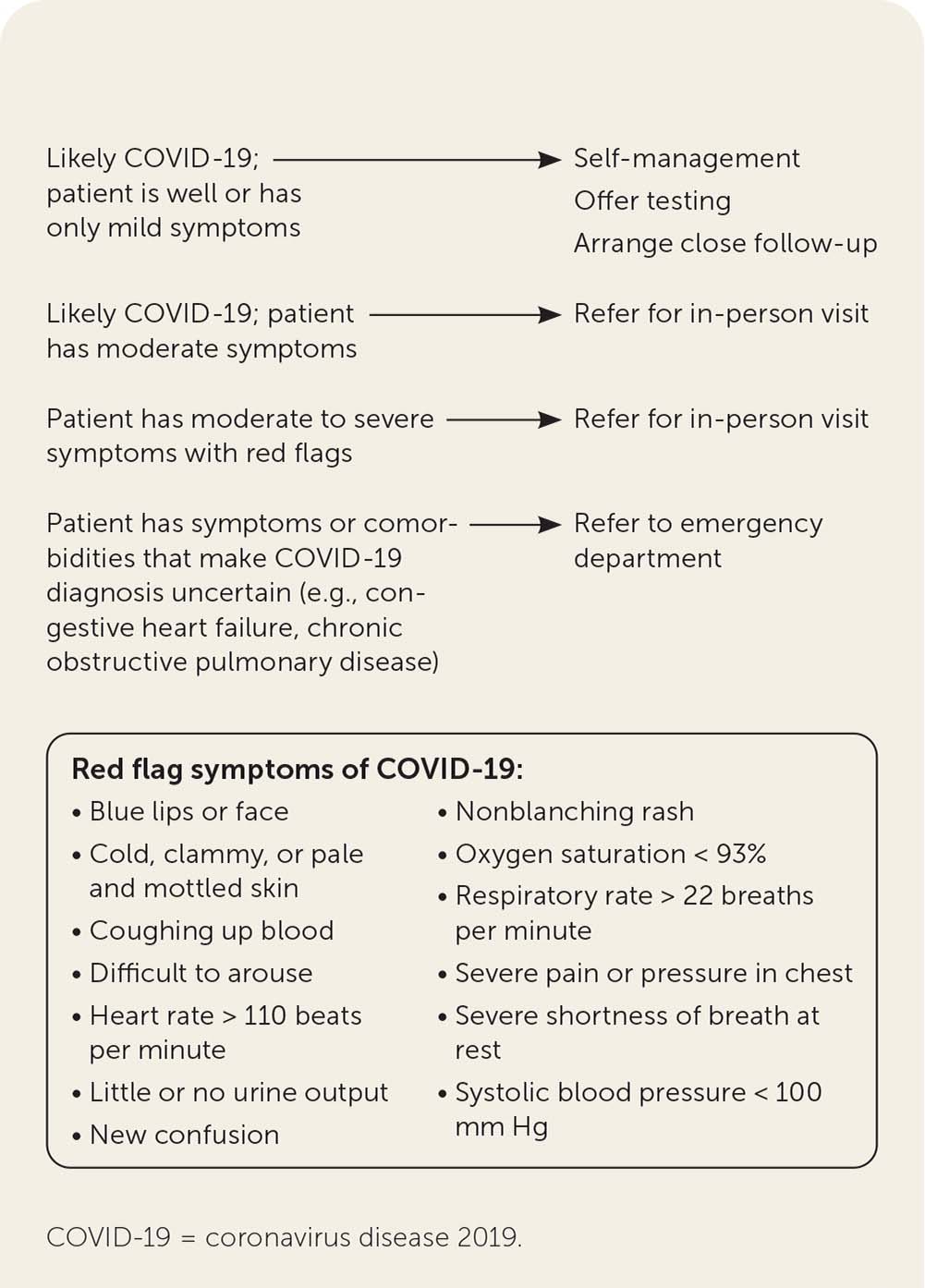
When possible, patients should be triaged via telehealth before they receive in-person care (Figure 1).21
Physicians should be aware of anchoring bias when considering a diagnosis of COVID-19. Given the wide variability in presentations, it should be considered as one among several potential etiologies.
The differential diagnosis includes:
○ Acute pulmonary edema
○ Acute respiratory distress syndrome
○ Chronic obstructive pulmonary disease exacerbation
○ Community-acquired pneumonia (bacterial or viral)
○ Influenza
○ Interstitial lung disease
○ Nonspecific viral upper respiratory tract infection
○ Opportunistic pulmonary infection
○ Pulmonary embolism
○ Streptococcal pharyngitis
SIGNS AND SYMPTOMS
The incubation period of SARS-CoV-2 is two to 14 days, although symptoms generally appear within five days of exposure.22
Common presenting symptoms include fever, dry cough, shortness of breath, and fatigue23; however, patients may have a wide range of symptoms representing a spectrum of mild to severe illness (Table 3).24–33
Moderate to severe anosmia and altered taste are commonly reported.28
Symptoms in children tend to be milder than in adults and may include fever, cough, and feeding difficulty (Arnaout R, et al., unpublished data, 2020).25
Pregnant and recently pregnant women are less likely to present with fever and myalgias compared with nonpregnant patients of reproductive age.34
A high proportion of patients with SARS-CoV-2 infection (about 40%) are asymptomatic, particularly younger patients.35
Multisystem inflammatory syndrome in children is thought to be related to COVID-19. Signs include prolonged fever and clinically severe illness with multiorgan involvement. The diagnosis is supported by laboratory confirmation of inflammation, evidence of recent SARS-CoV-2 infection, or exposure and no other obvious microbial cause of inflammation.36,37
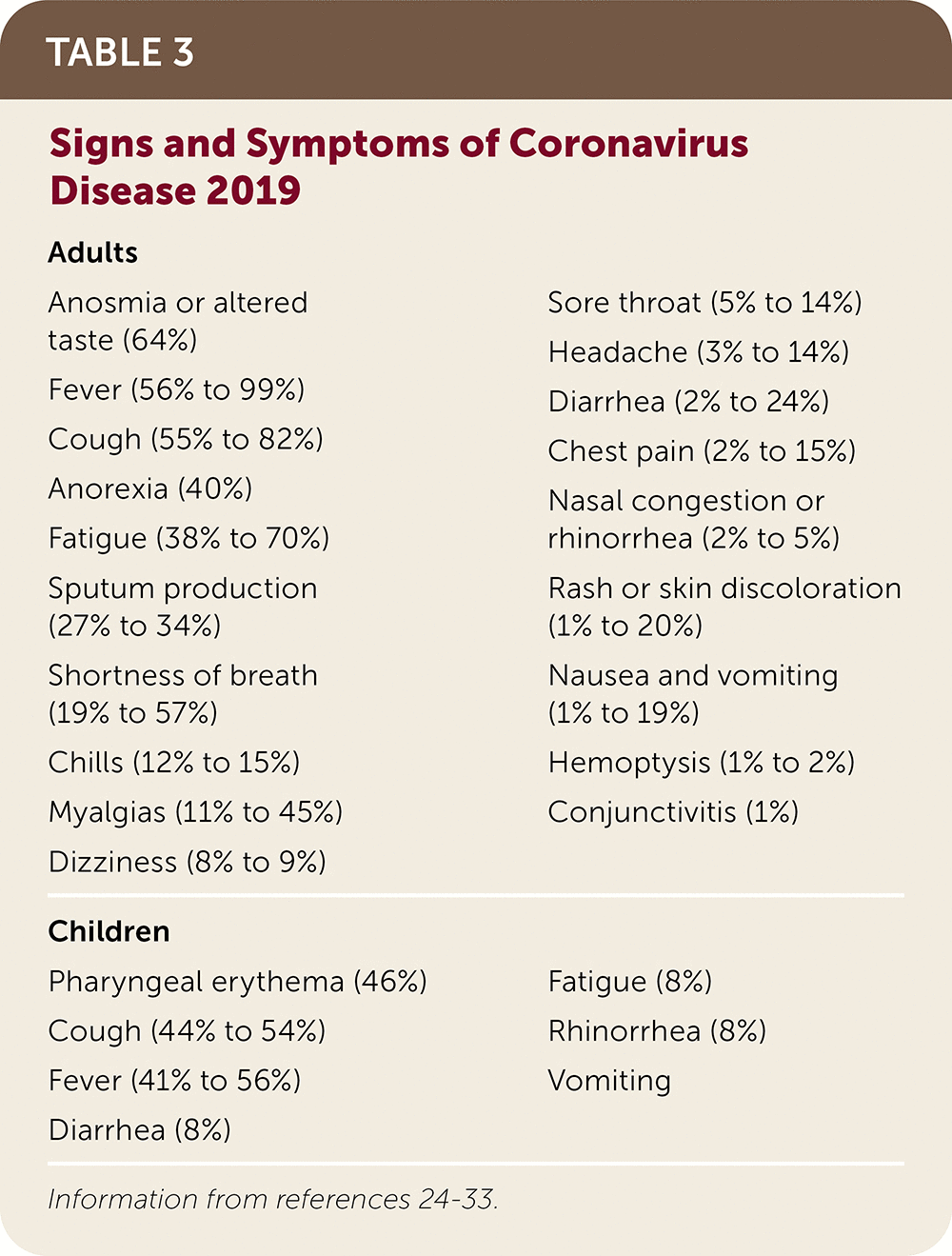
| Adults Anosmia or altered taste (64%) Fever (56% to 99%) Cough (55% to 82%) Anorexia (40%) Fatigue (38% to 70%) Sputum production (27% to 34%) Shortness of breath (19% to 57%) Chills (12% to 15%) Myalgias (11% to 45%) Dizziness (8% to 9%) Sore throat (5% to 14%) Headache (3% to 14%) Diarrhea (2% to 24%) Chest pain (2% to 15%) Nasal congestion or rhinorrhea (2% to 5%) Rash or skin discoloration (1% to 20%) Nausea and vomiting (1% to 19%) Hemoptysis (1% to 2%) Conjunctivitis (1%) |
| Children Pharyngeal erythema (46%) Cough (44% to 54%) Fever (41% to 56%) Diarrhea (8%) Fatigue (8%) Rhinorrhea (8%) Vomiting |
TESTING
There are multiple testing modalities for COVID-19 (Table 4).2,38–46 Acute infection should be confirmed with polymerase chain reaction testing using a nasopharyngeal swab.42 Antibody testing is most useful for epidemiologic purposes. 25 Point-of-care testing provides rapid results, but the reliability of these tests is not clear.41
The decision to test depends on variables such as availability of test kits and reagents, and implications for treatment, infection control, and public health.
In addition to patients with signs or symptoms of COVID-19, testing should be considered for patients who:
○ Have a known or suspected exposure (including neonates born to a mother diagnosed with COVID-19)
○ Will undergo a planned surgery or aerosol-generating procedure
○ Are undergoing chemotherapy
○ Are part of a group disproportionately affected by COVID-19, such as Black, Latino, Pacific Islander, or American Indian/Alaska Native communities.11,47
Testing asymptomatic people after an exposure is recommended five to seven days after the exposure based on the median viral incubation period. Testing should not occur for at least 48 hours after exposure. Asymptomatic contacts with negative test results still must quarantine for 14 days.11
Retesting can be considered to confirm disease resolution in immunocompromised patients if enough testing capacity exists.11
Radiographic findings in patients with COVID-19 may include45:
○ “Crazy paving” (i.e., ground-glass opacity with superimposed interlobular and intralobular septal thickening)
○ Dense consolidation
○ Ground-glass opacity
○ Multifocal ground-glass opacity
In hospitalized patients, the following laboratory tests can help assess prognosis and identify patients at risk of vascular and thrombotic complications 45:
○ Complete blood count with differential
○ C-reactive protein level
○ d-dimer assay
○ Lactate dehydrogenase level
○ Troponin level

| Type of test | Comments |
|---|---|
| Antibody test | The antibody test is most useful for epidemiologic purposes and in children with multisystem inflammatory syndrome to provide evidence of current or past COVID-19 infection.38 |
| It detects the presence of IgM and IgG antibodies to SARS-CoV-2, which emerge approximately five and 14 days after symptom onset, respectively (Wajnberg A, et al., unpublished data, 2020). A positive result can indicate prior infection or exposure, which is important for detecting infections with few or no symptoms. | |
| Early evidence suggests that most patients develop a vigorous antibody response to SARS-CoV-2,39 but it is not known if this correlates with resistance to future infection. | |
| Results should not be used as the sole basis on which to diagnose or exclude SARS-CoV-2 infection. | |
| A positive result may be due to current or past infection with non–SARS-CoV-2 coronavirus strains.40 | |
| Even tests with good specificity can yield more false-positive than true-positive results when population prevalence is low.41 | |
| PCR test | The PCR test detects the presence of coronavirus RNA and is used to confirm the diagnosis of COVID-19. |
| Positive results do not rule out bacterial infection or coinfection with other viruses. | |
| Sensitivity varies depending on time from symptom onset,42 so results should be interpreted based on clinical and epidemiologic factors.43 | |
| Nasopharyngeal swabs are somewhat more sensitive than oropharyngeal swabs. If both are collected, they may be combined and tested simultaneously in a single reaction to conserve reagents.44 | |
| Reliability is unknown because there is no standard diagnostic test,2 there are multiple test types, production methods are inconsistent, and implementation varies around the world. Extrapolating from data on PCR tests for influenza, the PCR rapid test for COVID-19 may be highly specific (90% to 95%), meaning a positive result is good at ruling in infection, but only moderately sensitive (50% to 70%), meaning a negative result does not reliably rule out infection (Arnaout R, et al., unpublished data, 2020).45 | |
| A negative result should be taken in the context of other symptoms, exposures, and the clinical presentation to guide recommendations. | |
| Point-of-care tests | Results are available within minutes. |
| Nucleic acid amplification testing targets part of the RdRp gene that is specific to SARS-CoV-2. | |
| Antigen testing uses antibodies and immunofluorescence to detect the presence of SARS-CoV-2. | |
| The reliability, sensitivity, and specificity of these tests are variable.41 |
Treatment
There are no evidence-based treatments for COVID-19 that are appropriate for use in the outpatient setting; management is supportive.45
The World Health Organization recommends that infants born to women with COVID-19 not be separated from their mothers, and that these mothers initiate and continue breastfeeding.48
Remote patient monitoring via telehealth can be used to detect red flag symptoms that warrant urgent assessment, such as21:
○ Fever above 100.4°F (38°C)
○ Heart rate greater than 100 beats per minute with new confusion
○ Oxygen saturation less than 95%
○ Respiratory rate greater than 20 breaths per minute
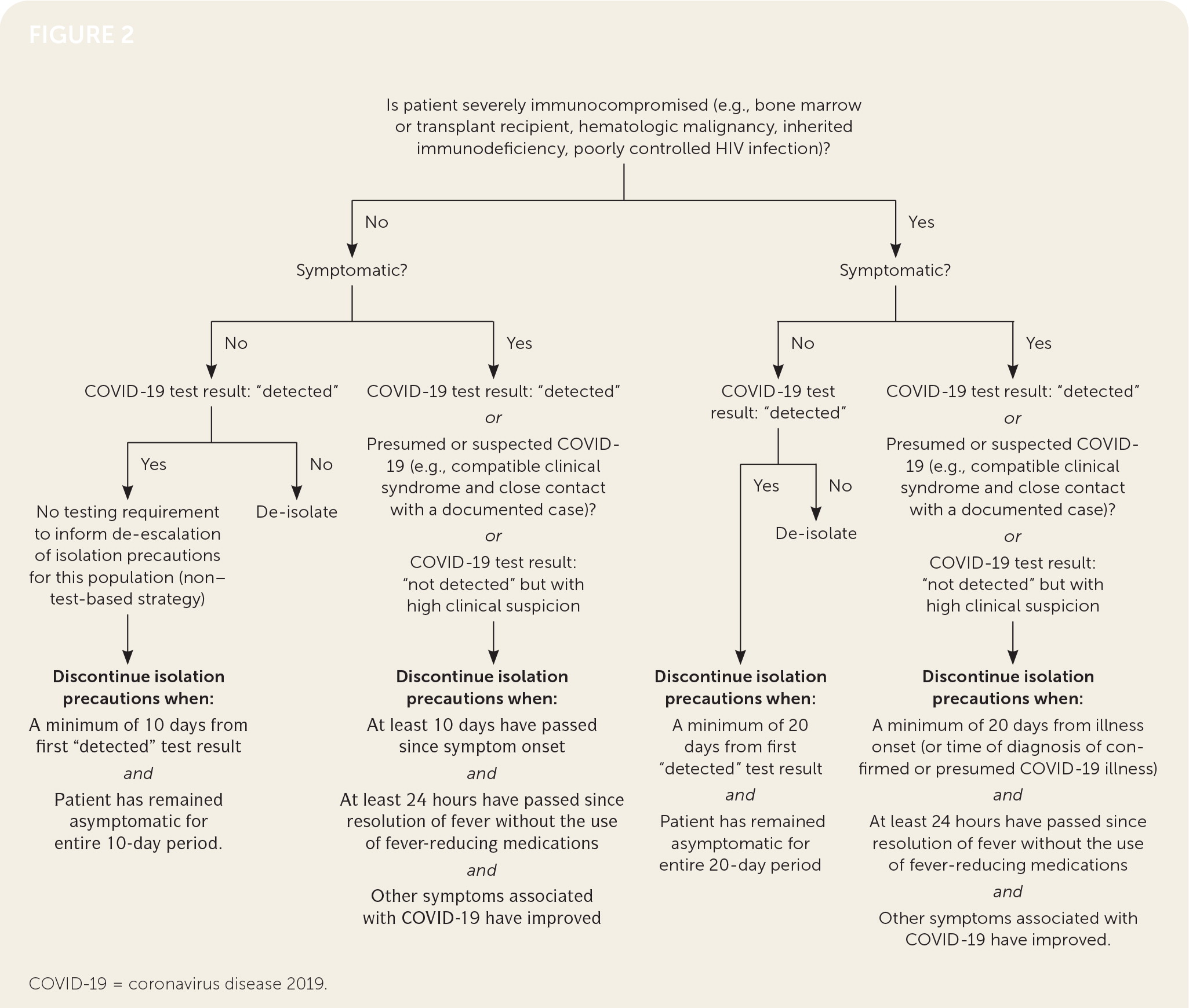
Figure 2 shows a protocol for discontinuing home isolation after COVID-19 diagnosis.11,49
DRUG THERAPY
In addition to supportive care, there are several investigational drugs being explored for the treatment of COVID-19 in hospitalized patients.
Dexamethasone, 6 mg per day for 10 days, significantly reduced mortality in hospitalized patients with COVID-19 who required supplemental oxygen (number needed to treat [NNT] = 29) or mechanical ventilation (NNT = 9), but not in hospitalized patients who did not require supplemental oxygen.50 Systemic corticosteroids are not recommended for outpatients or for hospitalized patients who do not require supplemental oxygen.51
Remdesivir (Veklury) was shown in a U.S. randomized trial to significantly reduce time to recovery (11 vs. 15 days) and to nonsignificantly reduce mortality (7.1% vs. 11.9%; P = .059) in hospitalized patients with COVID-19.52 A Chinese study found no significant benefit, but the study was underpowered.53 Remdesivir has not been studied in outpatients with nonsevere illness and is not recommended for this population.
Hydroxychloroquine (Plaquenil) has not been shown to have clear benefit in patients with mild to severe COVID-19 symptoms, or for postexposure prophylaxis (Horby P, et al., unpublished data, 2020).54–57
There is insufficient evidence to recommend the use of the following treatments for COVID-1945:
○ Azithromycin (Zithromax)
○ Convalescent plasma
○ Interferon
○ Ivermectin (Soolantra)
○ Lopinavir/ritonavir (Kaletra)
○ Ribavirin (Virazole)
○ Tocilizumab (Actemra)
REFERRAL, CONSULTATION, AND HOSPITALIZATION
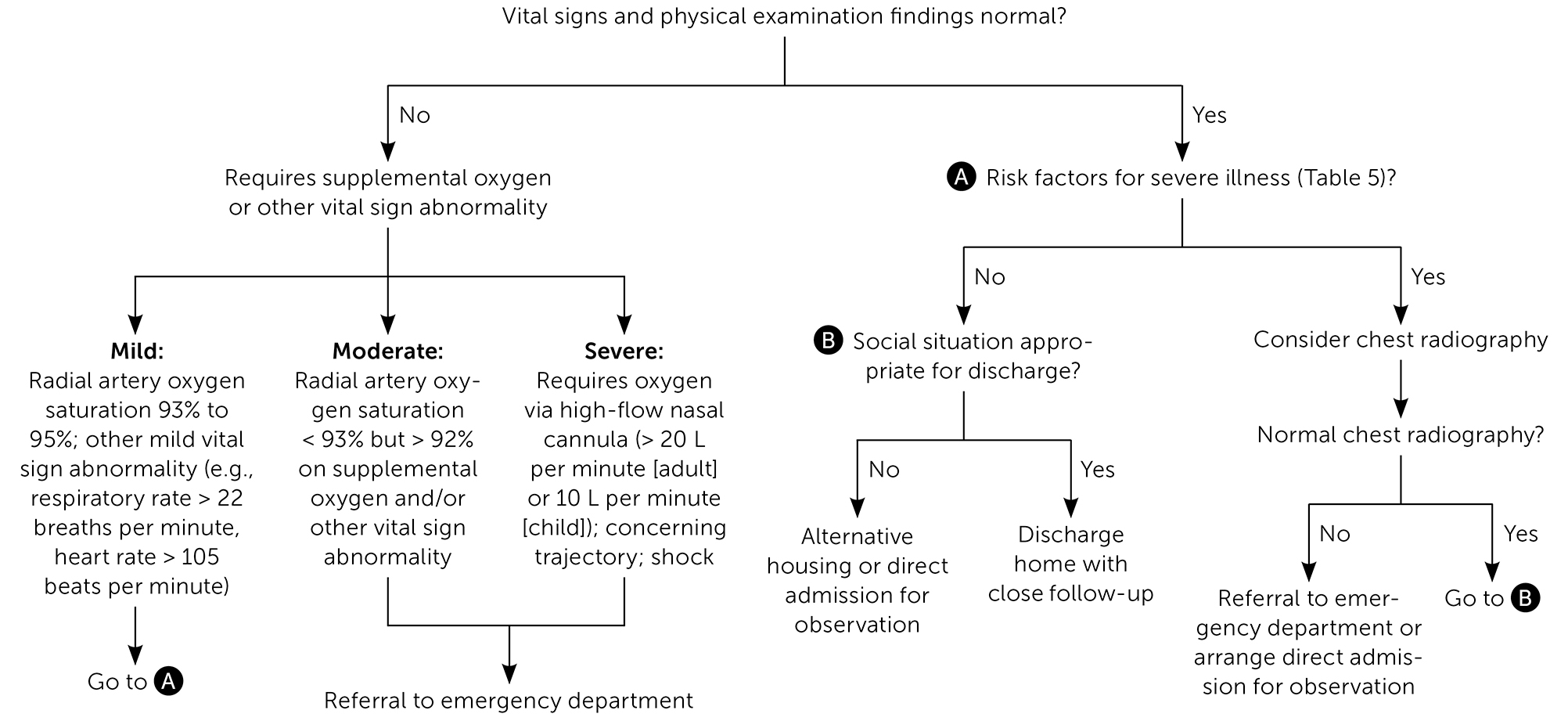
Prognosis
International data suggest that 85% of people with COVID-19 have only mild illness, whereas 14% have severe disease requiring hospitalization, including 5% of adults and 2% of children who require admission to an intensive care unit.60–62 Children tend to have a better prognosis than adults (Arnaout R, et al., unpublished data, 2020).62
The overall mortality rate from COVID-19 has been estimated to be 0.66% to 0.9%, although estimates are difficult because of the number of undiagnosed cases.63,64 Observed rates vary considerably (2.3% to 7.2%) depending on location and test availability. As of July 21, 2020, the Johns Hopkins Center for Health Security reported a U.S. case fatality rate of 3.7%.2 Case fatality rates increase with age.6,60,63,64
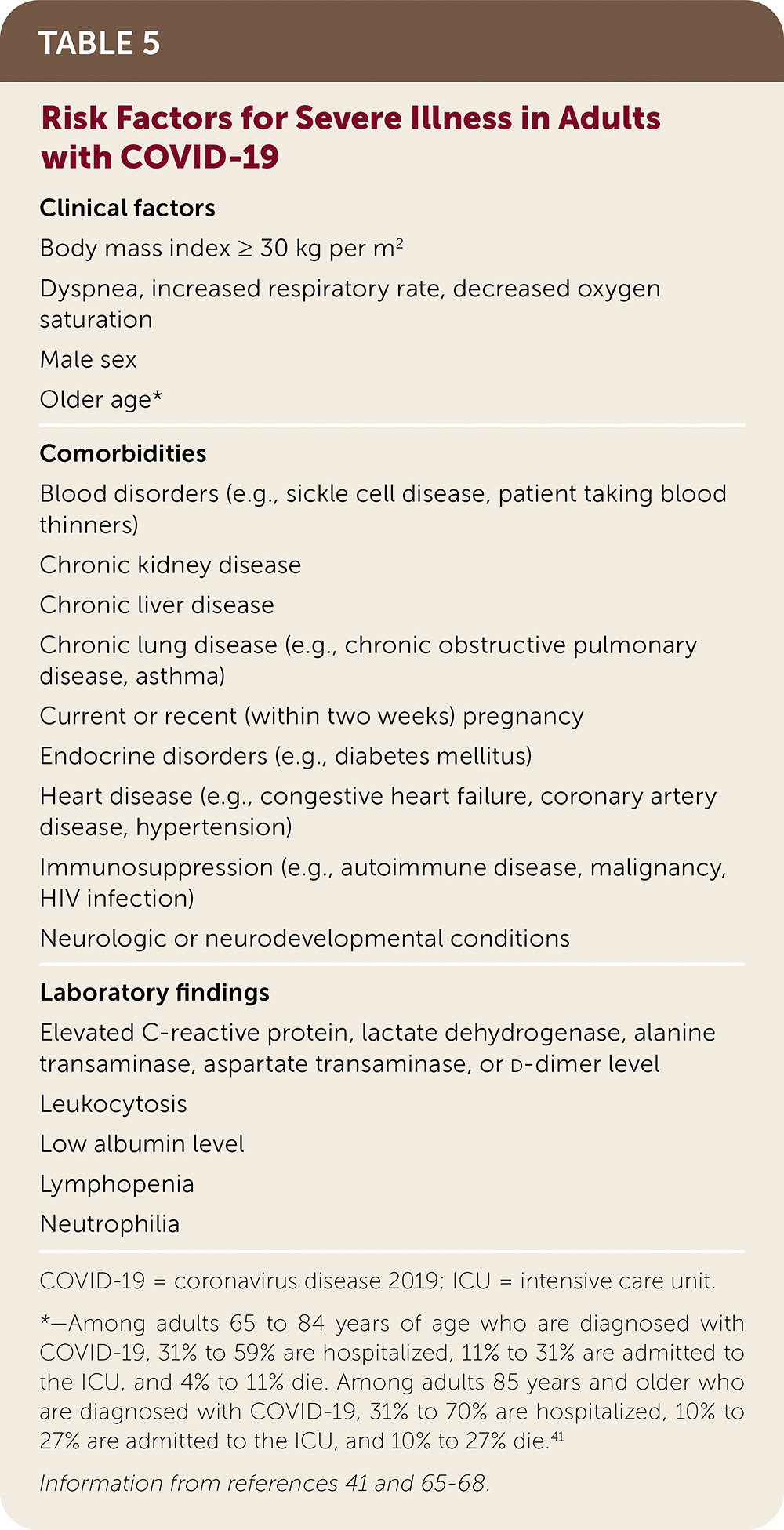
Predictors of more severe disease or death include clinical factors, comorbidities, and laboratory findings (Table 5).41,65–68 Common risk factors in hospitalized patients include66,67:
○ Cardiovascular disease
○ Chronic kidney disease
○ Chronic liver disease
○ Chronic obstructive pulmonary disease
○ Diabetes mellitus
○ Hypercholesterolemia
○ Hypertension
○ Malignancy
○ Obesity
○ Obstructive sleep apnea
In pregnant women, COVID-19 may increase the risk of complications such as preterm birth and preterm premature rupture of membranes. The risk of vertical transmission and risks to newborns are not well understood.34
A 10-item clinical prediction rule has been developed and externally validated to determine the prognosis of patients with COVID-19 (http://118.126.104.170).69 However, it requires imaging and extensive laboratory testing.
Acute complications of COVID-19 include pneumonia, acute respiratory distress syndrome, stroke, and arterial and venous thrombosis. Hospitalized patients may have debility 70 and mental health effects.71 Factors that may increase the risk of these complications include:
○ A diet with inadequate fruits and vegetables (Adams ML, et al., unpublished data, 2020)
○ Body mass index greater than 40 kg per m265
○ Sedentary lifestyle
○ Smoking
The long-term health effects of COVID-19 are under investigation.
Approximately 35% of people with COVID-19 have not returned to their previous level of health 14 to 21 days after diagnosis. These “long haulers” have a syndrome referred to as long COVID.72
Table 6 lists additional COVID-19 resources for clinicians.
| Clinical factors |
| Body mass index ≥ 30 kg per m2 |
| Dyspnea, increased respiratory rate, decreased oxygen saturation |
| Male sex |
| Older age* |
| Comorbidities |
| Blood disorders (e.g., sickle cell disease, patient taking blood thinners) |
| Chronic kidney disease |
| Chronic liver disease |
| Chronic lung disease (e.g., chronic obstructive pulmonary disease, asthma) |
| Current or recent (within two weeks) pregnancy |
| Endocrine disorders (e.g., diabetes mellitus) |
| Heart disease (e.g., congestive heart failure, coronary artery disease, hypertension) |
| Immunosuppression (e.g., autoimmune disease, malignancy, HIV infection) |
| Neurologic or neurodevelopmental conditions |
| Laboratory findings |
| Elevated C-reactive protein, lactate dehydrogenase, alanine transaminase, aspartate transaminase, or d-dimer level |
| Leukocytosis |
| Low albumin level |
| Lymphopenia |
| Neutrophilia |
| BMJ's COVID-19 hub https://www.bmj.com/coronavirus |
| European Centre for Disease Prevention and Control: contact tracing for COVID-19 https://www.ecdc.europa.eu/sites/default/files/documents/COVID-19-Contract-tracing-scale-up.pdf |
| Infectious Diseases Society of America: COVID-19 treatment guidelines https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management |
| National Institutes of Health: COVID-19 treatment guidelines https://www.covid19treatmentguidelines.nih.gov |
| World Health Organization: interim guidance on clinical management of COVID-19 https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected |
Data Sources: An initial evidence review was performed by the COVID Inquiry Group of the Oregon Health & Science University School of Medicine. Search terms included COVID, COVID-19, and SARD-CoV2. Answers were reviewed by two faculty reviewers. A subsequent literature review was performed using PubMed, Essential Evidence Plus, and the COVID-19 Daily Research Briefs on the AFP website. Search dates: March through September 2020.
This article was based on the Outpatient COVID-19 Reference Guide published in April 2020 by the Oregon Health & Science University (OHSU). The project lead was Jessica Hoyt; other contributors included Johanna Warren, MD; Dominic Caruso, MD; Anthony Cheng, MD; Eric Herman, MD; Debbie Lamberger, MPA; Craig McDougall, MD; Andy Barnett, MD; Daisuke Yamashita, MD; and Emily Barclay, MS. We wish to acknowledge the work of the OHSU COVID Lab Task Force and the OHSU School of Medicine COVID Inquiry Group: co-leads Frances Biagioli, MD, and Kam Pierce, MPA; core faculty members Heather Angier, PhD; Amy Chen, MD; Elizabeth Haney, MD; and Glenn Rodriguez, MD; core student group Hannah Bacheller, Eva Davis, Brett Lewis, Asma Lotfi, Lauren Raymond, Elizabeth Swanson, and Kanwarabijit Thind; contributing authors Brooke Bachelor, DO; Andrea Baron, MPH; Kathryn Bonuck, MS; Elizabeth Bower, MD; Annie Buckmaster, MD; Dominic Caruso, MD; Anthony Cheng, MD; Joshua Cohen, MD; Roopradha Datta, MPH; Shelby Lee Freed, FNP; Natalie Freitas, MD; Laurel Hallock-Koppelman, DNP; Cassandra Kasten-Arias; Roheet Kakaday, MD; Christine Le, MD; Justin Lewis, MD; Ingrid Lindquist, MD; Morgan Lisner, MD; Mustafa Mahmood, MD; Brittany McAdams, MD; Craig McDougall, MD; John Mitchell, MD; Laurence Moore, MD; Cassie Mullen, MD; Ryan Nesbit, MD; Kristen Otto, MD; Gina Phillipi; Megan Quinlan, MD; Sean Robinson, MD; Whitney Roper, MD; Eric Schmidt, MD; Linh Taylor; Erin Urbanowicz; Eric Wiser, MD; Jean Yau, MD; and Hirofumi Yoshida, MD.
Editor's Note: This article has been updated to incorporate new information about personal protective equipment requirements and the two COVID-19 vaccines that received Emergency Use Authorization from the U.S. Food and Drug Administration in December 2020. —Kenny Lin, MD, MPH, deputy editor of AFP
