Am Fam Physician. 2022;105(4):388-396
Related Letter to the Editor: Doxycycline Preferred for the Treatment of Chlamydia
Patient information: See related handouts on chlamydia, written by the authors of this article, and on gonorrhea, which has been adapted from a previously published AFP article.
Author disclosure: No relevant financial relationships.
Infections caused by Chlamydia trachomatis and Neisseria gonorrhoeae are increasing in the United States. Because most infections are asymptomatic, screening is key to preventing complications such as pelvic inflammatory disease and infertility and decreasing community and vertical neonatal transmission. All sexually active people with a cervix who are younger than 25 years and older people with a cervix who have risk factors should be screened annually for chlamydial and gonococcal infections. Sexually active men who have sex with men should be screened at least annually. Physicians should obtain a sexual history free from assumptions about sex partners or practices. Acceptable specimen types for testing include vaginal, endocervical, rectal, pharyngeal, and urethral swabs, and first-stream urine samples. Uncomplicated gonococcal infection should be treated with a single 500-mg dose of intramuscular ceftriaxone in people weighing less than 331 lb (150 kg). Preferred chlamydia treatment is a seven-day course of doxycycline, 100 mg taken by mouth twice per day. All nonpregnant people should be tested for reinfection approximately three months after treatment or at the first visit in the 12 months after treatment. Pregnant patients diagnosed with chlamydia or gonorrhea should have a test of cure four weeks after treatment.
Chlamydia trachomatis and Neisseria gonorrhoeae are the most common sexually transmitted infections (STIs) in the United States and are required to be reported to state health departments. Between 2015 and 2019, reported chlamydial infections increased by 19%, and reported gonococcal infections increased by 53%.1 These bacteria commonly infect the urogenital, anorectal, and pharyngeal sites but can become disseminated to affect multiple organ systems. Untreated infections may lead to pelvic inflammatory disease; scarring of fallopian tubes, which can increase the risk of ectopic pregnancy; infertility; easier transmission of new HIV infection; and vertical neonatal transmission.2

| Clinical recommendation | Evidence rating | Comments |
|---|---|---|
| Sexually active adolescents and adults at increased risk of acquiring a sexually transmitted infection should receive behavioral counseling to reduce their risk.6 | B | Systematic review |
| Sexually active people 24 years and younger who have a cervix should be screened for chlamydial and gonococcal infections annually.2,7 | B | Systematic reviews |
| Doxycycline should be used to treat chlamydia in nonpregnant people.2 | A | Systematic review of randomized controlled trials |
| Nonpregnant people treated for chlamydial or gonococcal infections should be tested for reinfection three months after treatment.2 | C | Consensus opinion from clinical guidelines |
| All newborns should receive ocular erythromycin 0.5% ointment to prevent gonococcal ophthalmia neonatorum.31 | A | High certainty of substantial net benefit |
Risk Factors
Young people 15 to 24 years of age account for 61% of all newly diagnosed STIs.1 Racial and ethnic minorities, men who have sex with men (MSM), and transgender and gender diverse people are at higher risk of STIs. Inequitable access to health insurance and physicians, language barriers, and distrust of medical systems because of discrimination account for some of these disparities, independent of individual sexual behavior.3,4 Other risk factors are reviewed in Table 1.2

| Age < 25 years Current sexually transmitted infection* Engaging in transactional sex* Having a new sex partner Having a sex partner with a current sexually transmitted infection Having a sex partner with other current partners Having multiple sex partners Inconsistent condom use Personal history of a sexually transmitted infection* Substance use (risk factor for men who have sex with men)* |
Taking a thorough sexual history is important to identify overall risk of infection, as well as anatomic site-specific risk factors. Physicians should create supportive spaces where patients feel safe sharing information by using open-ended questions; avoiding assumptions regarding sexual preferences, practices, and gender/sex; and normalizing diverse sexual experiences. To obtain a complete sexual history, the five P’s (partners, practices, pregnancy attitudes, previous STIs, and protection from STIs) model can be used as outlined in Table 2.2,5

| General questions | What are your pronouns? Do you think of yourself as male, female, transgender, or something else? What sex were you assigned at birth? Are there any words you would like me to use when we talk about specific body parts? Have you been sexually active in the past 12 months? |
| Partners | What gender do your partners identify as? How many sex partners have you had in the past two months? Past 12 months? Is it possible that any of your partners in the past 12 months were sexually active with someone else while they were sexually active with you? |
| Practices | How do you have sex? What parts do you use? For instance, some people engage in oral, rectal, or vaginal/frontal receptive sex. |
| Pregnancy attitudes | Would you like to have (more) children? If so, when do you think that might be? How important is it to you to prevent pregnancy? |
| Previous STIs | Have you ever had any STIs? |
| Protection from STIs | How do you protect yourself from STIs and HIV? Have you ever injected drugs? Have you ever been tested for STIs? Testing for STIs is recommended. Is it okay to do testing today? |
Prevention
The U.S. Preventive Services Task Force (USPSTF) recommends behavioral counseling on condom use, communication strategies for safer sex, and problem solving with those at increased risk of STIs.6 Adolescents and adults diagnosed with an STI in the past year, people reporting irregular condom use, and those with multiple partners or with partners belonging to a high-risk group are at increased risk. Physicians should emphasize barrier protection as the best way to prevent STIs.2
Screening
The USPSTF and Centers for Disease Control and Prevention (CDC) recommend annual screening for chlamydial and gonococcal infections to prevent infertility and pelvic inflammatory disease in sexually active people 24 years and younger with a cervix and in older people with a cervix who have risk factors.2,7 The CDC also recommends at least annual screening for MSM based on their risk factors. Screening should include the pharynx, urethra, and rectum based on reported anatomic sites of exposure. After discussion with the patient, it may be necessary to screen those sites even without reported exposure because of underreporting of sexual practices.2 Table 3 summarizes screening recommendations for chlamydial and gonococcal infections.2,8 There are significant gaps in research as it pertains to screening transgender and gender diverse patients.9 The CDC recommends screening based on an individual’s current anatomy and sexual practices.2

| Population | Age | Timing | Notes |
|---|---|---|---|
| Cisgender, heterosexual men | Insufficient evidence to recommend screening in this population | As needed | Consider screening high-risk populations, such as adolescents, patients in correctional facilities, and patients seen in sexually transmitted infection clinics |
| Cisgender men presenting to adolescent and sexually transmitted infection clinics | Young males | No evidence-based interval recommendation | — |
| High-risk* cisgender women, high-risk* transgender men, and nonbinary people with a cervix | ≥25 years | As needed | Retest three months after treatment Consider rectal screening for chlamydial and gonococcal infections and pharyngeal screening for gonococcal infection based on sexual behaviors and exposure |
| Pregnant people | ≤24 years ≥25 years if high risk* | At first prenatal visit Retest in third trimester if patient is high risk* or had a sexually transmitted infection during pregnancy | Test of cure four weeks after treatment and retest within three months |
| Men in correctional facilities | < 30 years | At intake with opt-out screening | — |
| Sexually active, cisgender women, transgender men, and nonbinary people with a cervix | ≤24 years | Annually | Retest three months after treatment Consider rectal screening for chlamydial and gonococcal infections and pharyngeal screening for gonococcal infection based on sexual behaviors and exposure |
| Sexually active men who have sex with men | All ages | Annually or every three to six months if high risk* | Urethral, rectal, and pharyngeal screening for gonococcal infection, based on anatomic site of exposure |
| Transgender/gender diverse | Screen based on anatomy and site of exposure | As needed | — |
| Women in correctional facilities | ≤35 years | At intake with opt-out screening | — |
Screening for urogenital infections only and neglecting pharyngeal and rectal sites of exposure will miss a substantial proportion of chlamydial and gonococcal infections.10 In one study of women who engaged in oral or anal sex with men, the prevalence of pharyngeal gonorrhea was 3.5%; rectal gonorrhea, 4.8%; and rectal chlamydia, 11.8%.10 Pharyngeal and rectal screening may be offered to people with female anatomy based on sexual practices and shared decision-making.2 Current evidence for screening extra-genital sites is strongest for MSM. Urine-only screening in an STI clinic misses 83% of infections among MSM.11 They should be screened at each anatomic site of sexual exposure, regardless of condom use, at least annually.2 Routine testing for chlamydial infections of the oropharynx is not recommended, but many laboratories will test for gonococcal and chlamydial infections simultaneously.2 If oropharyngeal chlamydia is diagnosed, it should be treated to decrease the risk of transmission.2
Presentation
Most chlamydial and gonococcal infections are asymptomatic.8 Symptoms of infection are reviewed in Table 4.2 Because dysuria may be a symptom of chlamydial and gonococcal infections and causes leukocytes on urinalysis, women presenting with dysuria may be inaccurately diagnosed with a urinary tract infection if STI testing is not performed.12,13 In women at risk for STIs or with a negative urine culture, physicians can consider STI testing in those presenting with dysuria. A pelvic examination is not required for diagnosis and may not improve the diagnosis of chlamydia and gonorrhea beyond history and diagnostic testing.14 However, if pelvic inflammatory disease is suspected, a pelvic examination should be performed. The differential diagnosis of chlamydial and gonococcal infections is summarized in Table 5.2,15

| Anorectal Anal pruritus, constipation, pain with anorectal intercourse, rectal bleeding, rectal discharge, rectal pain, tenesmus Disseminated gonococcal infection Asymmetric polyarthralgia, chills, fever, oligoarticular septic arthritis, rash, tenosynovitis; rarely endocarditis, meningitis, perihepatitis Genitourinary (female anatomy) Dysuria; irregular vaginal bleeding, including postcoital bleeding; mucoid, mucopurulent, or purulent urethral or endocervical discharge; urethral pruritus; increased urinary frequency Genitourinary (male anatomy) Dysuria; mucoid, mucopurulent, or purulent urethral discharge; pain with ejaculation; unilateral testicular pain or swelling; urethral pruritus; urinary frequency Lymphogranuloma venereum Swollen, tender, unilateral, inguinal or femoral lymph node with a self-limited ulcer or papule; rectal bleeding, mucous rectal discharge, rectal pain, fever, tenesmus, and/or constipation Oropharyngeal* Cervical lymphadenitis, oropharyngeal exudates, sore throat Pelvic inflammatory disease Abnormal bleeding, cervical motion tenderness, dyspareunia, elevated erythrocyte sedimentation rate or C-reactive protein, fever > 100.9°F (38.3°C), uterine or adnexal tenderness, vaginal discharge Perihepatitis (Fitz-Hugh-Curtis syndrome) Fever, nausea and vomiting with normal to slightly elevated transaminase levels, pelvic pain, right upper quadrant abdominal pain Reactive arthritis (Reiter syndrome) Aseptic arthritis, conjunctivitis, urethritis |

| Neonatal conjunctivitis Moraxella catarrhalis Other Neisseria species Urethritis in patients with male genitalia Adenovirus Herpes simplex virus Mycoplasma Trichomonas Ureaplasma urealyticum Urinary tract infection Vaginitis, cervicitis, urethritis inpatients with female genitalia Atrophic vaginitis Bacterial vaginosis (Gardnerella, Mycoplasma, Ureaplasma) Candidiasis Herpes simplex virus Irritant or allergen exposure Physiologic discharge (including pregnancy) Trichomoniasis |
Diagnostic Testing
The CDC recommends using nucleic acid amplification testing (NAAT) for the diagnosis of gonococcal or chlamydial infections because it is the most sensitive.2 Specimens can be taken from a first-stream urine sample without urethral cleansing before collection.2 Clinician- or patient-collected vaginal or endocervical swabs are also acceptable specimens. Self-collected vaginal swabs are as sensitive as clinician-collected swabs and are preferred by patients.16,17 A recent meta-analysis showed that urine samples and vaginal and endocervical swabs have similar sensitivity.17 For patients with male genitalia, a patient- or clinician-collected urethral swab may also be obtained, although a urine specimen is preferred.2
In 2019, the U.S. Food and Drug Administration (FDA) approved the Aptima Combo 2 Assay and Xpert CT/NG, which use NAAT, for extragenital swabs of the throat and rectum.18 The binx health io CT/NG assay, Visby Medical Sexual Health Test NAAT, and Cepheid Xpert CT/NG (not a waived test by the Clinical Laboratory Improvement Amendments) are point-of-care tests for the diagnosis of chlamydial and gonococcal infections.19 Point-of-care testing provides same-day results, decreases loss to follow-up, and reduces overtreatment.20,21
Management
All people who test positive or report known exposure to C. trachomatis or N. gonorrhoeae should be treated. Patients and their partners should be advised to abstain from sex for seven days after completing a single-dose regimen or until the completion of a seven-day treatment course and resolution of symptoms.2 Nonpregnant people should be tested for reinfection approximately three months after treatment or at the first visit in the 12 months after treatment. Follow-up care recommendations are reviewed in Table 6.2,22,23

| Advise patients and their sex partners to abstain from sexual contact for seven days after completing a single-dose regimen or until the completion of a seven-day treatment course and resolution of symptoms. |
| Avoid routine test of cure in nonpregnant people treated with CDC-recommended drug regimens, except for those with pharyngeal gonococcal infections, who should be retested seven to 14 days after treatment. |
| CDC-recommended drug regimens, except for those with pharyngeal gonococcal infections, who should be retested seven to 14 days after treatment. |
| Perform repeat testing for reinfection three months after completing treatment or at the first visit in the 12 months after treatment. |
| Retest all infected pregnant people four weeks after completing treatment. |
| Test for HIV and syphilis in anyone diagnosed with chlamydial or gonococcal infections. |
Patients presenting clinically with nongonococcal urethritis can be treated empirically at the time of evaluation while diagnostic testing is pending. Cervicitis should be treated presumptively in those younger than 25 years or those at high risk of infection if NAAT is not available or follow-up is uncertain.
CHLAMYDIAL TREATMENT
Although spontaneous clearance of chlamydial infections is possible, people with positive test results should always be treated.24 Because of increasing macrolide resistance, the recommended treatment for non-pregnant people is now doxycycline, 100 mg, twice per day for seven days.2 Physicians may alternatively choose to treat patients with a single 1-g dose of azithromycin, especially when adherence to a multidose regimen may be a concern.2 Treatment regimens are reviewed in Table 7.2,22,23
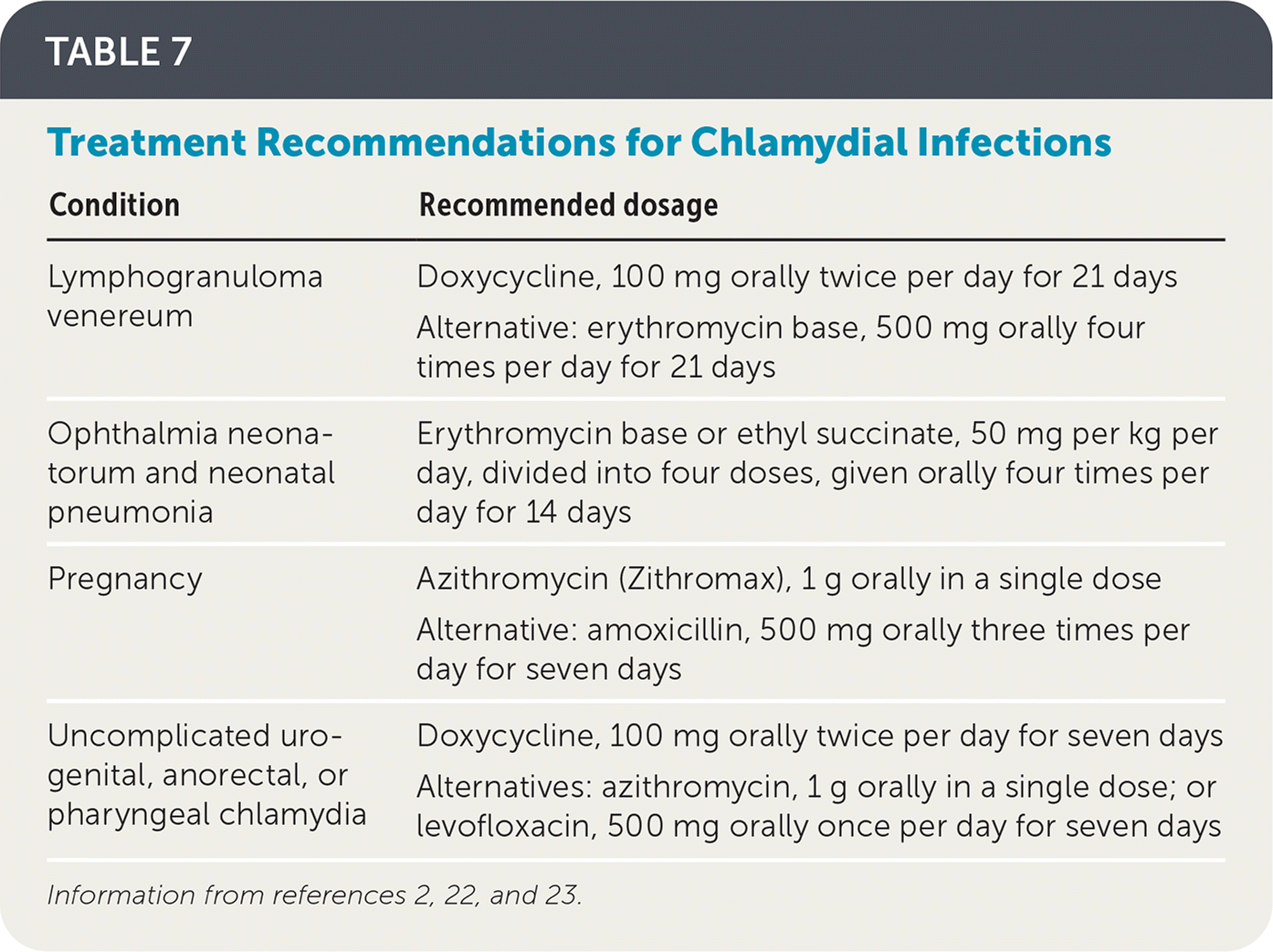
| Condition | Recommended dosage |
|---|---|
| Lymphogranuloma venereum | Doxycycline, 100 mg orally twice per day for 21 days Alternative: erythromycin base, 500 mg orally four times per day for 21 days |
| Ophthalmia neonatorum and neonatal pneumonia | Erythromycin base or ethyl succinate, 50 mg per kg per day, divided into four doses, given orally four times per day for 14 days |
| Pregnancy | Azithromycin (Zithromax), 1 g orally in a single dose Alternative: amoxicillin, 500 mg orally three times per day for seven days |
| Uncomplicated urogenital, anorectal, or pharyngeal chlamydia | Doxycycline, 100 mg orally twice per day for seven days Alternatives: azithromycin, 1 g orally in a single dose; or levofloxacin, 500 mg orally once per day for seven days |
GONOCOCCAL TREATMENT
In 2018, more than one-half of cases of gonococcal infection were estimated to be resistant to at least one drug, leading the CDC to change treatment recommendations to higher doses of ceftriaxone25 (Table 82,15,25). Azithromycin is no longer a recommended therapy for nonpregnant individuals because of an observed sevenfold increase in gonococcal resistance between 2013 and 2018.25

| Infection | Recommended treatment |
|---|---|
| Disseminated gonococcal infection | Consult infectious disease specialist2 |
| Exposed asymptomatic neonate | Ceftriaxone (Rocephin), 20 to 50 mg per kg intravenously or intramuscularly in a single dose, not to exceed 250 mg Alternative if unable to receive ceftriaxone: cefotaxime (Claforan), 100 mg per kg intravenously or intramuscularly in a single dose |
| Ophthalmia neonatorum prophylaxis | Erythromycin 0.5% ophthalmic ointment, single application in each eye at birth |
| Pharyngeal gonorrhea | People weighing < 331 lb (150 kg): ceftriaxone, 500 mg intramuscularly in a single dose People weighing ≥ 331 lb: ceftriaxone, 1 g intramuscularly in a single dose Consult infectious disease specialist if ceftriaxone cannot be administered If chlamydia coinfection, add doxycycline, 100 mg orally twice per day for seven days If pregnant and chlamydia coinfection, add azithromycin, 1 g orally in a single dose |
| Uncomplicated urogenital or anorectal gonorrhea | People weighing < 331 lb: ceftriaxone, 500 mg intramuscularly in a single dose People weighing ≥ 331 lb: ceftriaxone, 1 g intramuscularly in a single dose Alternative if ceftriaxone is not available: gentamicin, 240 mg intramuscularly in a single dose, plus azithromycin, 2 g orally in a single dose; or cefixime (Suprax), 800 mg orally in a single dose If chlamydia has not been excluded, add doxycycline, 100 mg orally twice per day for seven days If pregnant and chlamydia has not been excluded, add azithromycin, 1 g orally in a single dose |
UNRESOLVED SYMPTOMS
Most treatment failures are caused by reinfection from sex partners who have not received adequate treatment, rather than treatment failure from antimicrobial resistance.2 If symptoms do not resolve or a test is persistently positive in a situation in which reinfection seems unlikely (i.e., the patient has reported no new sexual contact and is taking medication as prescribed), an infectious disease specialist and local health department should be consulted in case of possible antimicrobial resistance.2
PARTNER EVALUATION AND EXPEDITED PARTNER THERAPY
If seeking care in person is not possible, expedited partner therapy is a strategy in which sex partners of a person diagnosed with a chlamydial or gonococcal infection within the past 60 days can be prescribed treatment without being seen by the physician. This strategy is supported by the American Academy of Family Physicians.2,26 If the diagnosed person has not had a sex partner in the past 60 days, the most recent sex partner can be offered treatment. Sex partners with symptoms should be referred for evaluation and treatment.2 Laws in 46 states permit expedited partner therapy.27 Because recommended gonococcal treatment is based on intramuscular administration of medication, every effort should be made to see partners of infected patients in person for treatment and testing for other STIs.28 If permissible by state law and the partner is highly unlikely to receive care, partners of those with gonococcal infections may be treated with a single dose of cefixime (Suprax), 800 mg orally, with the addition of 100 mg of oral doxycycline twice per day for seven days if chlamydial infection was not excluded.28 Written instructions should be given to patients to convey to their partners how to take the medication, warnings about side effects and allergies, when to seek medical care, and STI education.2 The best evidence for use of expedited partner therapy services is for male partners of women with gonococcal or chlamydial infections.27 The risk of missing concomitant infections in MSM requires a more nuanced discussion, but these patients may be offered expedited partner therapy through shared decision-making.28
LYMPHOGRANULOMA VENEREUM
Lymphogranuloma venereum is caused by a C. trachomatis serovar and can become invasive and cause colorectal fistulas and strictures. Treatment should be started presumptively at the initial visit to prevent complications if there is clinical suspicion for lymphogranuloma venereum.2 Partners should be evaluated and treated empirically with a non–lymphogranuloma venereum chlamydial infection regimen.2
PREGNANCY
Gonococcal and chlamydial infections in pregnancy are associated with increased risks, including preterm birth, premature rupture of membranes, stillbirth, low-birth-weight infants, and neonatal infection.29,30 Pregnant patients should be screened as outlined in Table 3.2,8 Those at high risk of infection should be screened again in the third trimester.2 Anyone diagnosed with a gonococcal or chlamydial infection during pregnancy should have a test of cure approximately four weeks after treatment, at three months after diagnosis, and in their third trimester.2
NEONATAL INFECTIONS
The prevalence of perinatal gonococcal infections is 0.2 to 0.4 cases per 100,000 live births.31 The USPSTF recommends universal prophylaxis with ocular erythromycin 0.5% ointment to prevent gonococcal ophthalmia neonatorum. The risk of infection without prophylaxis is 30% to 40%, and it can cause blindness as early as 24 hours after birth.31 N. gonorrhoeae can also cause septic arthritis, meningitis, rhinitis, vaginitis, urethritis, pneumonia, and skin infections in neonates.2 Asymptomatic newborns exposed to gonorrhea at birth from an untreated birthing parent should be swabbed for infection at the conjunctiva, oropharynx, vagina, and rectum and presumptively treated for gonorrhea.2
Neonates are at high risk of contracting an infection if chlamydia is untreated in pregnancy.2,32 Infants exposed during birth do not need to receive chlamydial-specific prophylactic antibiotics but should be monitored clinically for symptoms.2 Ophthalmia neonatorum presents a few days to several weeks after birth with eyelid edema, discharge, and ocular congestion.2,32 Chlamydial infections of the eye are not prevented by prophylactic erythromycin ointment.32 Unlike trachoma, which is a chronic infection spread through close contact, clothes, and flies, ophthalmia neonatorum does not result in scarring and blindness. Diagnosis of ophthalmia neonatorum can be made by swabbing the conjunctiva for culture, direct fluorescence antibody testing, or NAAT.2 The recommended treatment is oral erythromycin.2 There should be close follow-up because a second course may be required.2 C. trachomatis can also cause neonatal pneumonia. Infants present between two and 19 weeks of age with a staccato cough, tachypnea, rhinorrhea, and rales.2,32 Exposed infants are at high risk; if they present with pneumonia, they should be treated empirically for chlamydial infection while awaiting test results from culture, direct fluorescence antibody testing (lower sensitivity), or NAAT (not FDA approved for the nasopharynx).2,32
MANAGEMENT DURING THE COVID-19 PANDEMIC
Disparities in STI testing have been more pronounced due to reallocation of resources for SARS-CoV-2 testing and decreased testing due to social distancing and stay-at-home orders.3,19 However, telemedicine use has increased during the COVID-19 pandemic and is well-suited for STI screening because physical examination is not essential for diagnosis or treatment.14,33 At-home C. trachomatis and N. gonorrhoeae self-testing kits are not FDA approved; however, multiple studies have found that when patients are instructed by a physician via telemedicine, self-collected swabs at home will diagnose cases similarly to office-collected samples, with increased volume of testing offsetting a slightly lower test sensitivity.19,34–36 Physicians can safely incorporate home-based testing and treatment into telehealth practice.
This article updates previous articles on this topic by Mishori, et al.23; Mayor, et al.15; Miller37; and Miller.38
Data Sources: The U.S. Preventive Services Task Force, Cochrane Database of Systematic Reviews, Essential Evidence Plus, Centers for Disease Control and Prevention, the U.S. Food and Drug Administration, and American Academy of Family Physicians websites were reviewed for relevant publications. A PubMed search was conducted using the terms Neisseria gonorrhoeae, Chlamydia trachomatis, diagnosis, and treatment for the past 10 years including English language, meta-analysis, randomized controlled trials, reviews, and systematic reviews. Search dates: January 28, 2021; February 14, 2021; March 30, 2021; July 25, 2021; and November 27, 2021.