
Am Fam Physician. 2022;105(5):558-560
Author disclosure: No relevant financial relationships.
Key Points for Practice
• Topical corticosteroids are the most effective topical agent for psoriasis plaques.
• Topical calcineurin inhibitors, vitamin D analogues, and tazarotene are effective and can limit chronic topical corticosteroid use.
• When topical vitamin D analogues are prescribed, avoid vitamin D supplementation, avoid use over large areas, and avoid sun exposure shortly after application.
From the AFP Editors
Psoriasis is a chronic, multisystem inflammatory disorder affecting one out of every 30 people in the United States. In 2021, the American Academy of Dermatology and National Psoriasis Foundation updated their recommendations on topical therapies for patients with mild to moderate psoriasis.
Topical Corticosteroids
Corticosteroids are rated by potency into seven classes (Table 1), with class 1 being the most potent and class 7 being the least potent. Initial treatment with class 2 to 5 agents has the best evidence of benefit, with a number needed to treat (NNT) of 3 for 50% improvement in four weeks and an NNT of 2 with 12 weeks of treatment. Lower-potency products are best used on the face, forearms, and intertriginous areas to avoid adverse effects. Higher-potency agents are reserved for thick, chronic plaques. All corticosteroid products can be used in combination with systemic treatments.
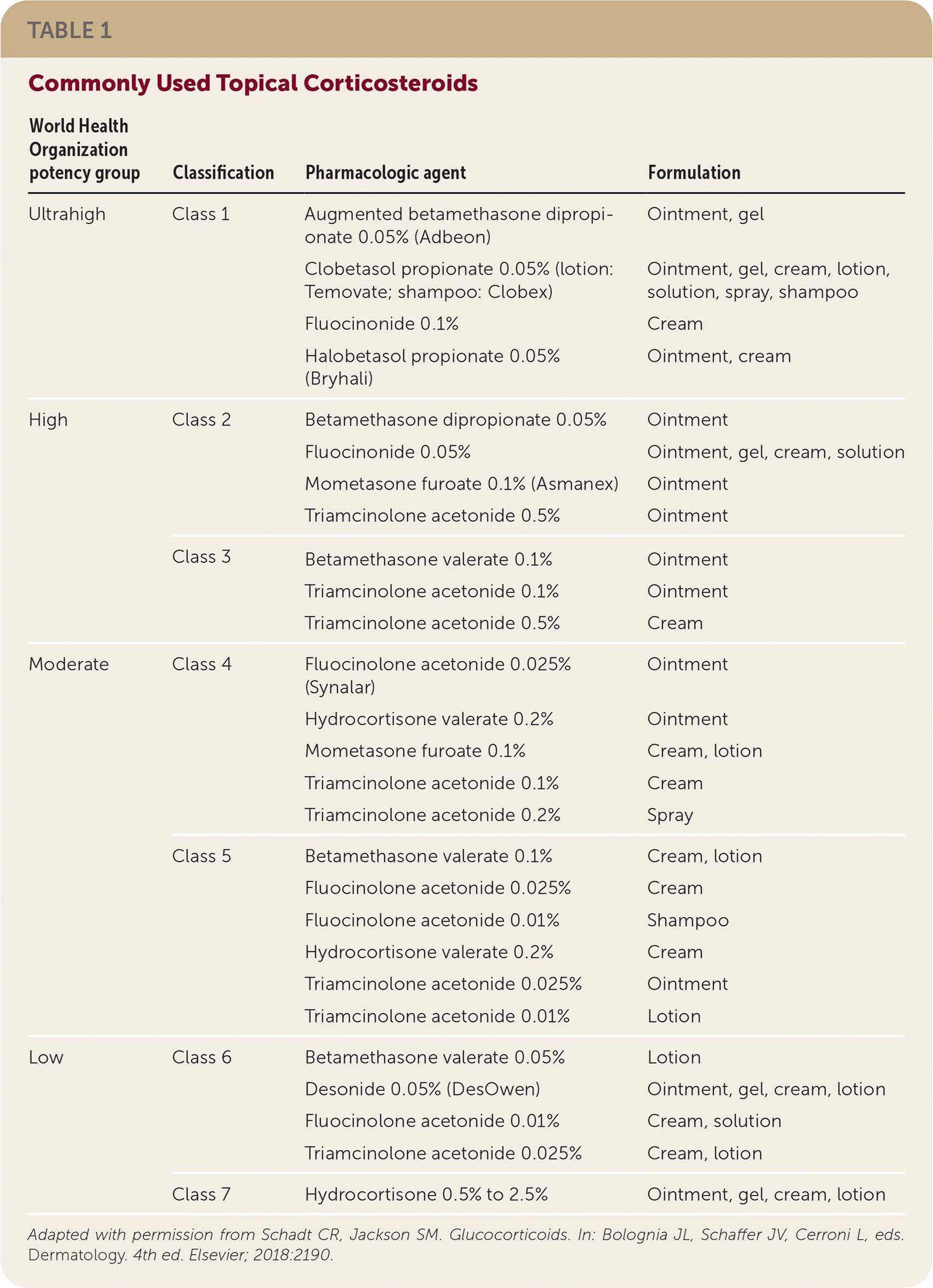
| World Health Organization potency group | Classification | Pharmacologic agent | Formulation |
|---|---|---|---|
| Ultrahigh | Class 1 | Augmented betamethasone dipropionate 0.05% (Adbeon) | Ointment, gel |
| Clobetasol propionate 0.05% (lotion: Temovate; shampoo: Clobex) | Ointment, gel, cream, lotion, solution, spray, shampoo | ||
| Fluocinonide 0.1% | Cream | ||
| Halobetasol propionate 0.05% (Bryhali) | Ointment, cream | ||
| High | Class 2 | Betamethasone dipropionate 0.05% | Ointment |
| Fluocinonide 0.05% | Ointment, gel, cream, solution | ||
| Mometasone furoate 0.1% (Asmanex) | Ointment | ||
| Triamcinolone acetonide 0.5% | Ointment | ||
| Class 3 | Betamethasone valerate 0.1% | Ointment | |
| Triamcinolone acetonide 0.1% | Ointment | ||
| Triamcinolone acetonide 0.5% | Cream | ||
| Moderate | Class 4 | Fluocinolone acetonide 0.025% (Synalar) | Ointment |
| Hydrocortisone valerate 0.2% | Ointment | ||
| Mometasone furoate 0.1% | Cream, lotion | ||
| Triamcinolone acetonide 0.1% | Cream | ||
| Triamcinolone acetonide 0.2% | Spray | ||
| Class 5 | Betamethasone valerate 0.1% | Cream, lotion | |
| Fluocinolone acetonide 0.025% | Cream | ||
| Fluocinolone acetonide 0.01% | Shampoo | ||
| Hydrocortisone valerate 0.2% | Cream | ||
| Triamcinolone acetonide 0.025% | Ointment | ||
| Triamcinolone acetonide 0.01% | Lotion | ||
| Low | Class 6 | Betamethasone valerate 0.05% | Lotion |
| Desonide 0.05% (DesOwen) | Ointment, gel, cream, lotion | ||
| Fluocinolone acetonide 0.01% | Cream, solution | ||
| Triamcinolone acetonide 0.025% | Cream, lotion | ||
| Class 7 | Hydrocortisone 0.5% to 2.5% | Ointment, gel, cream, lotion |
Skin atrophy, purpura, and striae over application areas are common adverse effects, especially with higher potency products. Rosacea and acne exacerbations can be caused by corticosteroids. Hypothalamic pituitary adrenal axis suppression is rare, even with extensive high-potency treatment. Rebound exacerbation can occur after abrupt withdrawal. Tapering over several weeks is recommended after desired clinical improvement.
Calcineurin Inhibitors
Tacrolimus (Protopic) and pimecrolimus (Elidel) offer the opportunity to avoid the effects of chronic corticosteroid use, particularly on areas with thinner skin such as the face and intertriginous areas. Burning and pruritus are common after application but improve with continued use. Both treatments commonly cause flushing after ingestion of alcohol. Both contain U.S. Food and Drug Administration boxed warnings for risk of malignancy based on their systemic use, but no evidence has suggested increased risk when used topically.
Vitamin D Analogues
Calcipotriene (cream: Dovonex; ointment: Calcitrene) and calcitriol (Vectical) improve psoriatic lesions alone or in combination with topical corticosteroids; combination therapy is superior to either agent as monotherapy. Common combination strategies include vitamin D analogues on weekdays and topical corticosteroids on weekends or high-potency corticosteroids in the morning and vitamin D analogues in the evening. Oral vitamin D supplementation is not recommended while using calcipotriene or calcitriol to avoid hypercalcemia.
Adverse effects are primarily local and include burning, pruritus, peeling, and erythema. Hypercalcemia and parathyroid hormone suppression are rare unless applied to more than 30% of body surface area or combined with oral supplements. Apply vitamin D analogues after periods of sunlight or ultraviolet light exposure to prevent drug inactivation.
Tazarotene
Tazarotene (Tazorac) is a topical retinoid that has long been used for treatment of mild to moderate psoriasis, and 12 weeks of use increases treatment success, with an NNT of 7. Research supports synergy between tazarotene and topical corticosteroids, increasing the duration of treatment effect and total remission time. Treatment is also successful in combination with ultraviolet light.
Erythema, burning, and pruritus are common adverse effects, especially at higher drug concentrations. Using a cream formulation, combined with moisturizer or topical corticosteroid and applied every other day, can limit adverse effects. Avoid using tazarotene in patients who are pregnant or trying to become pregnant.
Other Topical Agents
Emollients reduce desquamation and itching when using corticosteroids. They can be safely applied several times per day.
Salicylic acid products reduce scaling and soften plaques. Combining salicylic acid with corticosteroids or calcineurin inhibitors increases drug penetration and effectiveness. Avoid combining salicylic acid with vitamin D analogues because the low pH will cause inactivation. Avoid use in patients with renal or hepatic impairment or when treatment areas exceed 20% body surface area to avoid salicylate toxicity.
Hydrocarbons such as anthralin and coal tar products are also effective for mild to moderate psoriasis. Anthralin 0.1% is similarly effective as calcipotriene, with risk of burning, perilesional erythema, and skin staining, especially when applying anthralin for longer than two hours. Patients should avoid application to the face or other sensitive areas.
Coal tar appears to be even more effective than calcipotriene. Common adverse effects include folliculitis, contact dermatitis, and phototoxicity. Avoid coal tar products in patients who are pregnant, trying to become pregnant, or lactating.
Alternative Medicine
Indigo naturalis is a topical herbal medication that appears to be more effective than placebo in treating nail psoriasis. Indigo naturalis can stain skin and clothing and should be prepared by a pharmacist specializing in integrative medicine.
Consider aloe vera for mild psoriasis if patients request a natural therapy. Although one study showed dramatic improvement from regular use, another showed no benefit. Avoid use in patients with allergies to the lily family, including onion and garlic.
Topical St. John's wort may be beneficial in mild psoriasis but is not recommended because of poor standardization of products. Photosensitivity after use is common, and concomitant phototherapy should be avoided. Omega-3 fish oil oral supplements may be beneficial adjuncts to other therapies.
Lifestyle Modifications
Although patients with psoriasis have higher incidence rates of celiac disease, gluten-free diets are helpful only in patients with confirmed celiac disease.
Stress reduction techniques can be recommended as adjunctive therapy despite little evidence of benefit due to lack of harm.
Editor's Note: The NNTs were calculated by the author using data provided in the guideline.
Although many dermatology guidelines have little applicability to primary care, this guideline focuses on the topical therapies family physicians employ. These recommendations parallel the recommendations from a previous American Family Physician article on psoriasis (https://www.aafp.org/afp/2013/0501/p626.html) and add more context and evidence when available.—Michael J. Arnold, MD, Contributing Editor
Guideline source: American Academy of Dermatology and National Psoriasis Foundation
Evidence rating system used? Yes
Systematic literature search described? Yes
Guideline developed by participants without relevant financial ties to industry? No
Recommendations based on patient-oriented outcomes? Yes
Published source: J Am Acad Dermatol. February 2021;84(2):432–470.
