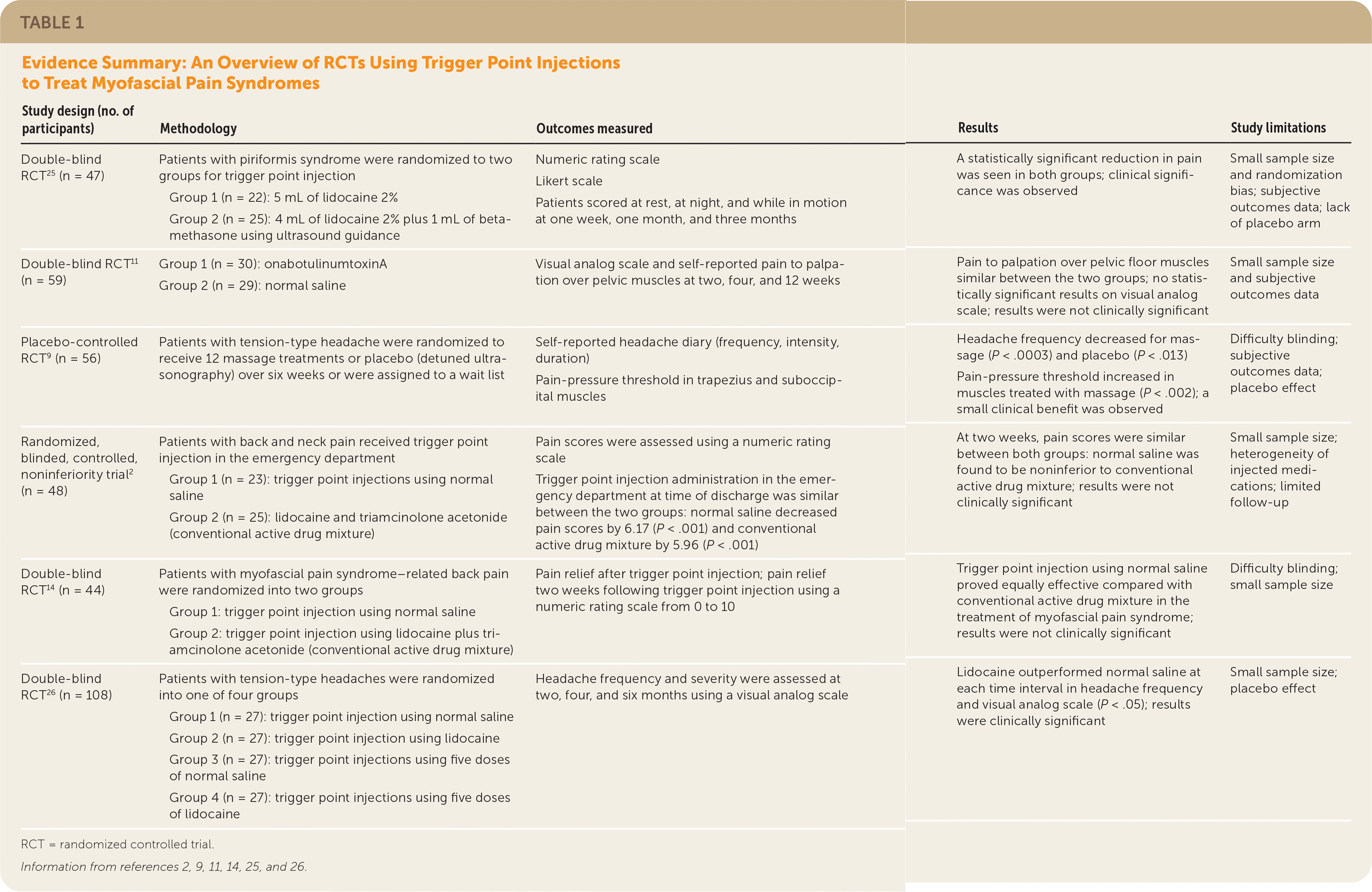
| Study design (no. of participants) | Methodology | Outcomes measured | Results | Study limitations |
|---|---|---|---|---|
| Double-blind RCT 25 (n = 47) | Patients with piriformis syndrome were randomized to two groups for trigger point injection Group 1 (n = 22): 5 mL of lidocaine 2% Group 2 (n = 25): 4 mL of lidocaine 2% plus 1 mL of betamethasone using ultrasound guidance | Numeric rating scale Likert scale Patients scored at rest, at night, and while in motion at one week, one month, and three months | A statistically significant reduction in pain was seen in both groups; clinical significance was observed | Small sample size and randomization bias; subjective outcomes data; lack of placebo arm |
| Double-blind RCT 11 (n = 59) | Group 1 (n = 30): onabotulinumtoxinA Group 2 (n = 29): normal saline | Visual analog scale and self-reported pain to palpation over pelvic muscles at two, four, and 12 weeks | Pain to palpation over pelvic floor muscles similar between the two groups; no statistically significant results on visual analog scale; results were not clinically significant | Small sample size and subjective outcomes data |
| Placebo-controlled RCT9 (n = 56) | Patients with tension-type headache were randomized to receive 12 massage treatments or placebo (detuned ultrasonography) over six weeks or were assigned to a wait list | Self-reported headache diary (frequency, intensity, duration) Pain-pressure threshold in trapezius and suboccipital muscles | Headache frequency decreased for massage (P < .0003) and placebo (P < .013) Pain-pressure threshold increased in muscles treated with massage (P < .002); a small clinical benefit was observed | Difficulty blinding; subjective outcomesdata; placebo effect |
| Randomized, blinded, controlled, noninferiority trial2 (n = 48) | Patients with back and neck pain received trigger point injection in the emergency department Group 1 (n = 23): trigger point injections using normal saline Group 2 (n = 25): lidocaine and triamcinolone acetonide (conventional active drug mixture) | Pain scores were assessed using a numeric rating scale Trigger point injection administration in the emergency department at time of discharge was similar between the two groups: normal saline decreased pain scores by 6.17 (P < .001) and conventional active drug mixture by 5.96 (P < .001) | At two weeks, pain scores were similar between both groups: normal saline was found to be noninferior to conventional active drug mixture; results were not clinically significant | Small sample size; heterogeneity of injected medications; limited follow-up |
| Double-blind RCT 14 (n = 44) | Patients with myofascial pain syndrome–related back pain were randomized into two groups Group 1: trigger point injection using normal saline Group 2: trigger point injection using lidocaine plus triamcinolone acetonide (conventional active drug mixture) | Pain relief after trigger point injection; pain relief two weeks following trigger point injection using a numeric rating scale from 0 to 10 | Trigger point injection using normal saline proved equally effective compared with conventional active drug mixture in the treatment of myofascial pain syndrome; results were not clinically significant | Difficulty blinding; small sample size |
| Double-blind RCT 26 (n = 108) | Patients with tension-type headaches were randomized into one of four groups Group 1 (n = 27): trigger point injection using normal saline Group 2 (n = 27): trigger point injection using lidocaine Group 3 (n = 27): trigger point injections using five doses of normal saline Group 4 (n = 27): trigger point injections using five doses of lidocaine | Headache frequency and severity were assessed at two, four, and six months using a visual analog scale | Lidocaine outperformed normal saline at each time interval in headache frequency and visual analog scale (P < .05); results were clinically significant | Small sample size; placebo effect |