
Am Fam Physician. 2023;107(3):238-246
Related Letter to the Editor: Enhanced Screening for People Living With HIV Infection
Patient information: See related handout on osteoporosis, written by the authors of this article.
Author disclosure: No relevant financial relationships.
Osteoporosis affects 10.2% of adults older than 50 years and is expected to increase to 13.6% by 2030. Osteoporotic fractures, specifically hip fractures, significantly affect morbidity, mortality, and quality of life. Screening for osteoporosis with dual energy x-ray absorptiometry should be considered for all women 65 years and older or women who are postmenopausal with clinical risk factors. The Bone Health and Osteoporosis Foundation recommends screening men 70 years and older and men with clinical risk factors; however, the U.S. Preventive Services Task Force did not find sufficient evidence to support routine screening in men. Osteoporosis can be diagnosed by a T-score of −2.5 or less or the presence of a fragility fracture. All patients with osteoporosis should be counseled on weight-bearing exercise, smoking cessation, moderation of alcohol intake, and calcium and vitamin D supplementation. Treatment of osteoporosis is influenced by the patient’s fracture risk, the effectiveness of fracture risk reduction, and medication safety. Patients at high risk of fracture should consider treatment with antiresorptive therapy, including bisphosphonates and denosumab. Anabolic agents such as teriparatide, abaloparatide, and romosozumab should be considered for patients at very high risk or with previous vertebral fractures.
In 2010, the incidence of osteoporosis was 10.2% in people older than 50 years and is expected to reach 13.6% by 2030 based on projected population demographics.1 Approximately 2 million to 3 million osteoporotic fractures occur annually in the United States, which can significantly affect morbidity, mortality, and quality of life.2 Hip fractures can be especially debilitating and have a one-year mortality risk of 21% to 24%.3 Adequate detection and management can decrease risks and associated comorbidities.

| Recommendation | Sponsoring organization |
|---|---|
| Do not use DEXA screening for osteoporosis in women younger than 65 years or men younger than 70 years with no risk factors. | American Academy of Family Physicians |
| Do not routinely repeat DEXA more often than once every two years. | American College of Rheumatology |
What Are the Current Screening Recommendations for Osteoporosis?
Dual energy x-ray absorptiometry (DEXA) is recommended for all women 65 years and older or women who are postmenopausal and younger than 65 years with clinical risk factors for osteoporotic fracture. Although the U.S. Preventive Services Task Force (USPSTF) does not recommend routine screening for men, the Bone Health and Osteoporosis Foundation recommends screening for men 70 years and older.
EVIDENCE SUMMARY
The USPSTF, the Bone Health and Osteoporosis Foundation, and the American Association of Clinical Endocrinologists/American College of Endocrinology (AACE/ACE) recommend bone mineral density (BMD) measurement by DEXA for all women 65 years and older (Table 1).4–6 Women younger than 65 years who are postmenopausal should undergo an osteoporosis risk assessment, including an evaluation of clinical risk factors and fracture risk assessment to determine the need for DEXA4–6 (Table 24,5). The most used assessment tool is the Fracture Risk Assessment Tool (FRAX; https://www.sheffield.ac.uk/FRAX); however, there is some controversy about the underestimation of risk in some races (Black, Asian, Hispanic) and a relatively low sensitivity. FRAX estimates a 10-year risk of major osteoporotic and hip fractures and should be used as part of the overall risk assessment.7–10 In a clinical trial, using the FRAX score to determine the need for DEXA improved the percentage of patients receiving therapy at one year and decreased hip fractures by 28% (P = .002).11 The USPSTF did not find benefit in routine BMD screening in men; however, the Bone Health and Osteoporosis Foundation recommends screening men 70 years and older and men with high clinical risk.5,6 The Male Osteoporosis Risk Estimation Score uses age, weight, and a history of chronic obstructive pulmonary disease to assess risk, may help determine the need for DEXA in men, and is more sensitive but less specific than the FRAX score.10,12 The American Academy of Family Physicians supports the USPSTF screening recommendations for osteoporosis.
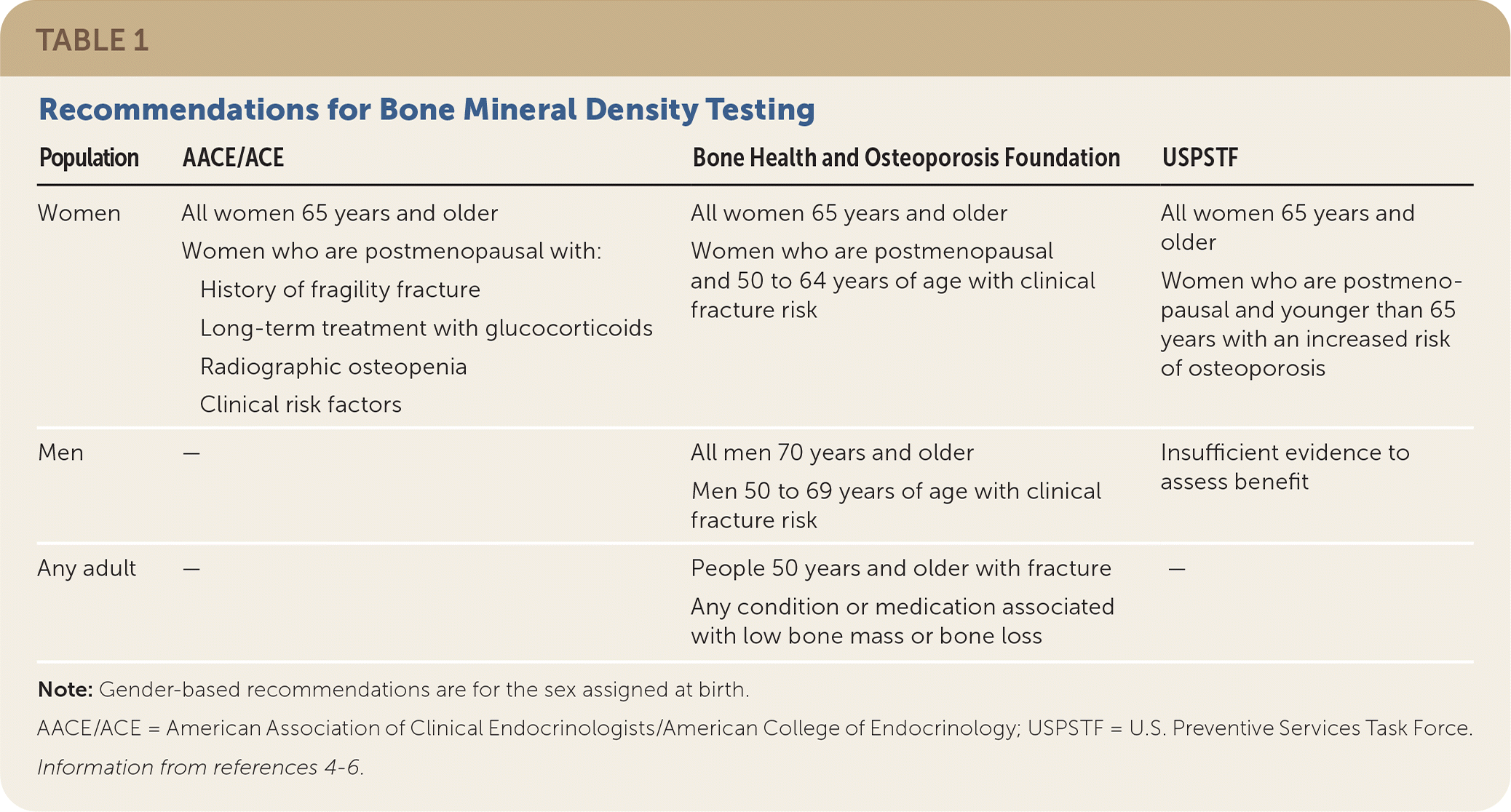
| Population | AACE/ACE | Bone Health and Osteoporosis Foundation | USPSTF |
|---|---|---|---|
| Women | All women 65 years and older Women who are postmenopausal with: History of fragility fracture Long-term treatment with glucocorticoids Radiographic osteopenia Clinical risk factors | All women 65 years and older Women who are postmenopausal and 50 to 64 years of age with clinical fracture risk | All women 65 years and older Women who are postmenopausal and younger than 65 years with an increased risk of osteoporosis |
| Men | — | All men 70 years and older Men 50 to 69 years of age with clinical fracture risk | Insufficient evidence to assess benefit |
| Any adult | — | People 50 years and older with fracture Any condition or medication associated with low bone mass or bone loss | — |
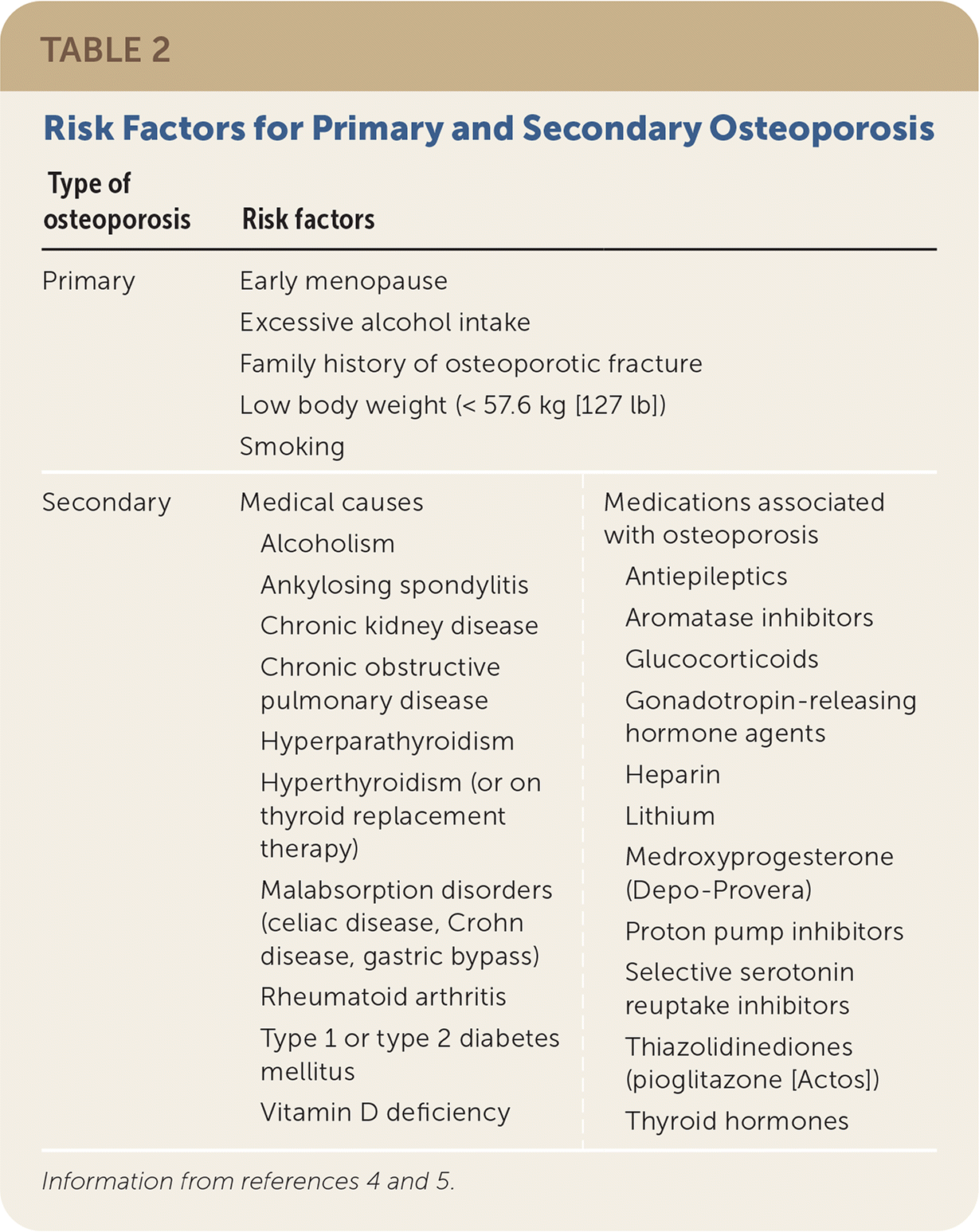
| Type of osteoporosis | Risk factors | |
|---|---|---|
| Primary | Early menopause Excessive alcohol intake Family history of osteoporotic fracture Low body weight (< 57.6 kg [127 lb]) Smoking | |
| Secondary | Medical causes Alcoholism Ankylosing spondylitis Chronic kidney disease Chronic obstructive pulmonary disease Hyperparathyroidism Hyperthyroidism (or on thyroid replacement therapy) Malabsorption disorders (celiac disease, Crohn disease, gastric bypass) Rheumatoid arthritis Type 1 or type 2 diabetes mellitus Vitamin D deficiency | Medications associated with osteoporosis Antiepileptics Aromatase inhibitors Glucocorticoids Gonadotropin-releasing hormone agents Heparin Lithium Medroxyprogesterone (Depo-Provera) Proton pump inhibitors Selective serotonin reuptake inhibitors Thiazolidinediones (pioglitazone [Actos]) Thyroid hormones |
How Is Osteoporosis Diagnosed?
Osteoporosis is diagnosed based on BMD (T-score of −2.5 or less on DEXA) or the presence of a fragility fracture or vertebral fracture.
EVIDENCE SUMMARY
Osteoporosis is diagnosed based on central DEXA BMD measurements at the hip and lumbar spine.6 BMD should be measured at the forearm (1/3 radius) when the hip and lumbar spine cannot be accurately measured due to structural changes (i.e., osteophytes).5 In women who are postmenopausal and men 50 years and older, BMD is classified using the World Health Organization diagnostic T-score criteria based on a young adult reference population (Table 3).5 The International Society of Clinical Densitometry recommends that ethnicity- or race-adjusted z scores (also adjusted for age or sex norms) should be used for patients 20 to 50 years of age with z scores of −2.0 or less for the expected range for age being diagnostic of osteoporosis.5
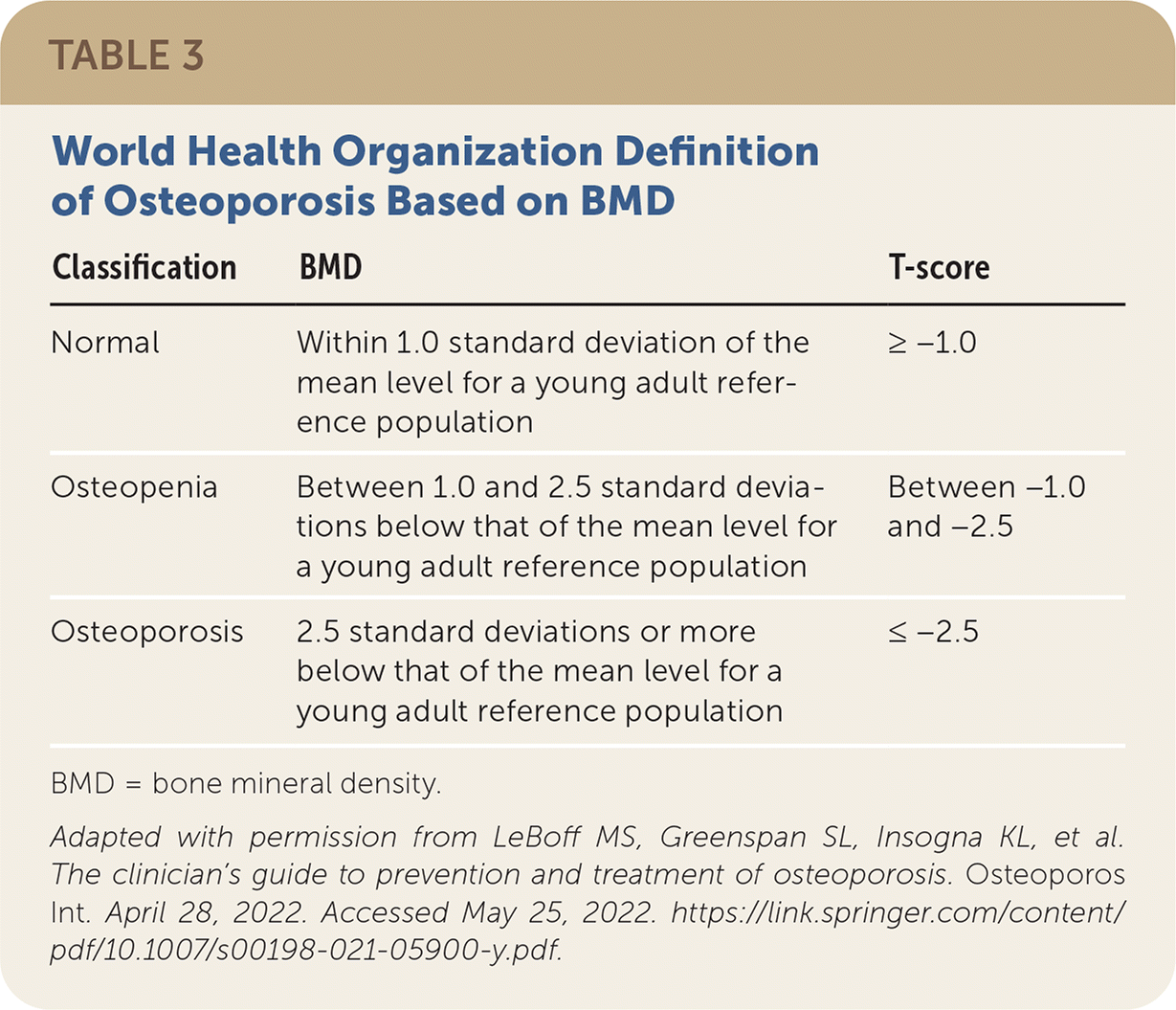
| Classification | BMD | T-score |
|---|---|---|
| Normal | Within 1.0 standard deviation of the mean level for a young adult reference population | ≥ −1.0 |
| Osteopenia | Between 1.0 and 2.5 standard deviations below that of the mean level for a young adult reference population | Between −1.0 and −2.5 |
| Osteoporosis | 2.5 standard deviations or more below that of the mean level for a young adult reference population | ≤ −2.5 |
Osteoporosis is also diagnosed based on the presence of a fragility or vertebral fracture, even in the absence of a BMD diagnosis.4,5 The AACE/ACE and National Bone Health Alliance extend osteoporosis diagnostic criteria to include patients with osteopenia and a 10-year risk of at least 20% for major osteoporotic fractures or at least 3% for hip fractures determined by the FRAX score.4
What Evaluation Should Be Performed Following an Osteoporosis Diagnosis?
To identify possible secondary causes of osteoporosis, an initial workup for most patients typically includes a complete blood count; complete metabolic panel; measurement of 25-hydroxyvitamin D, parathyroid hormone, and phosphate; and a 24-hour urine collection for calcium excretion.
EVIDENCE SUMMARY
Secondary osteoporosis may be present in healthy women who are postmenopausal; therefore, the AACE/ACE and the Bone Health and Osteoporosis Foundation recommend a standard evaluation that includes a complete blood count, complete metabolic panel, 25-hydroxyvitamin D, parathyroid hormone, phosphate, and a 24-hour urine collection for calcium and creatinine excretion.4,5 Further evaluation for secondary causes can be extensive and should be individualized based on a patient’s history, examination, and comorbid conditions (Table 2).4,5
Vertebral fractures indicate a high risk of future fractures and may change an individual’s risk, diagnostic classification, and clinical management. Patients meeting indications for vertebral imaging should be referred for high-quality imaging with dedicated spine radiography or vertebral fracture assessment performed at the same time as DEXA (Table 4).4,5
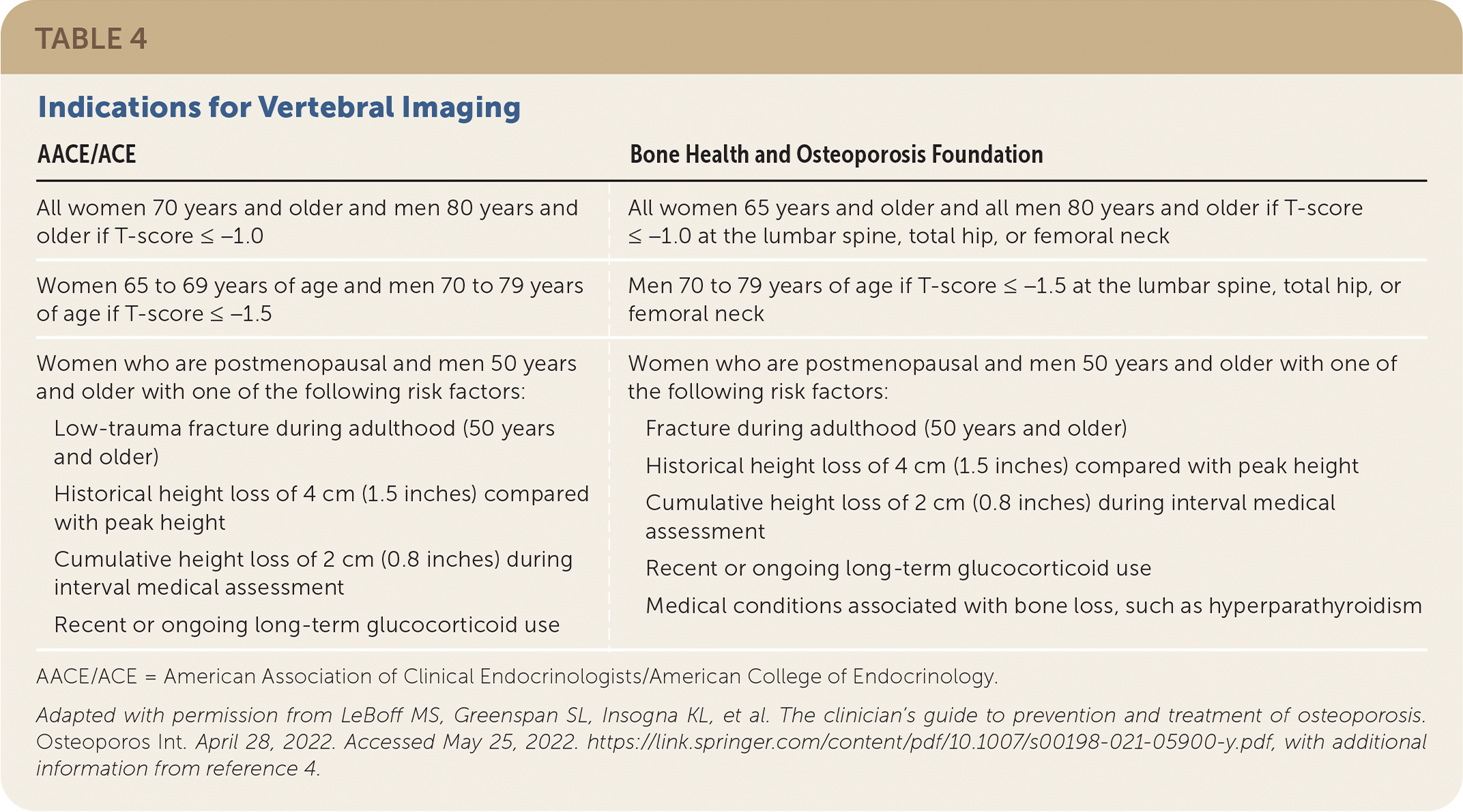
| AACE/ACE | Bone Health and Osteoporosis Foundation |
|---|---|
| All women 70 years and older and men 80 years and older if T-score ≤ −1.0 | All women 65 years and older and all men 80 years and older if T-score ≤ −1.0 at the lumbar spine, total hip, or femoral neck |
| Women 65 to 69 years of age and men 70 to 79 years of age if T-score ≤ −1.5 | Men 70 to 79 years of age if T-score ≤ −1.5 at the lumbar spine, total hip, or femoral neck |
| Women who are postmenopausal and men 50 years and older with one of the following risk factors: Low-trauma fracture during adulthood (50 years and older) Historical height loss of 4 cm (1.5 inches) compared with peak height Cumulative height loss of 2 cm (0.8 inches) during interval medical assessment Recent or ongoing long-term glucocorticoid use | Women who are postmenopausal and men 50 years and older with one of the following risk factors: Fracture during adulthood (50 years and older) Historical height loss of 4 cm (1.5 inches) compared with peak height Cumulative height loss of 2 cm (0.8 inches) during interval medical assessment Recent or ongoing long-term glucocorticoid use Medical conditions associated with bone loss, such as hyperparathyroidism |
What Nonpharmacologic Interventions Are Effective for Osteoporosis?
Patients diagnosed with osteopenia or osteoporosis should receive calcium and vitamin D supplementation based on their age and vitamin D levels. Weight-bearing exercise, physical therapy, fall prevention, smoking cessation, and moderation of alcohol intake are also recommended.
EVIDENCE SUMMARY
In one meta-analysis, calcium or vitamin D supplementation alone did not decrease the risk of hip fracture; however, the combination showed a 19% reduction in hip fracture.13 Guidelines recommend calcium and vitamin D as adjunctive therapy with other osteoporosis medications.4,5,14 Dietary counseling can encourage adequate calcium intake (1,000 mg per day for men 50 to 70 years of age; 1,200 mg per day for women older than 50 years and men older than 70 years).5 Recommended vitamin D intake is at least 800 to 1,000 IU per day for individuals older than 50 years.5 Vitamin D deficiency should be treated to maintain a level of at least 30 ng per mL.4,5 Weight-bearing exercise and physical therapy can improve BMD and prevent falls. Tobacco and alcohol use can decrease BMD and increase fracture risk; therefore, patients should be counseled on cessation strategies.4,5 For patients with fractures, multidisciplinary care coordination with a fracture liaison service or similar program is recommended to ensure appropriate evaluation and management.5
Which Patients Should Receive Pharmacologic Treatment for Osteoporosis?
Patients at high or very high risk of fracture benefit from pharmacologic management. This includes patients with a previous fragility fracture, osteoporosis on DEXA, or osteopenia with a high risk of fracture.
EVIDENCE SUMMARY
The decision to treat is determined based on fracture risk assessment (Table 54,14,15). Clinical practice guidelines from the Endocrine Society, the AACE/ACE, and the Bone Health and Osteoporosis Foundation support the use of pharmacologic treatment for patients with a previous hip or vertebral fracture; patients with a T-score of −2.5 or less at the femoral neck, total hip, or lumbar spine; or patients with a T-score between −1 and −2.5 and a 10-year risk of at least 20% for major osteoporotic fracture or at least 3% for hip fracture based on the FRAX tool.4,5,14,15 Patients with recent or multiple fractures are at very high risk and may warrant more aggressive therapy.5
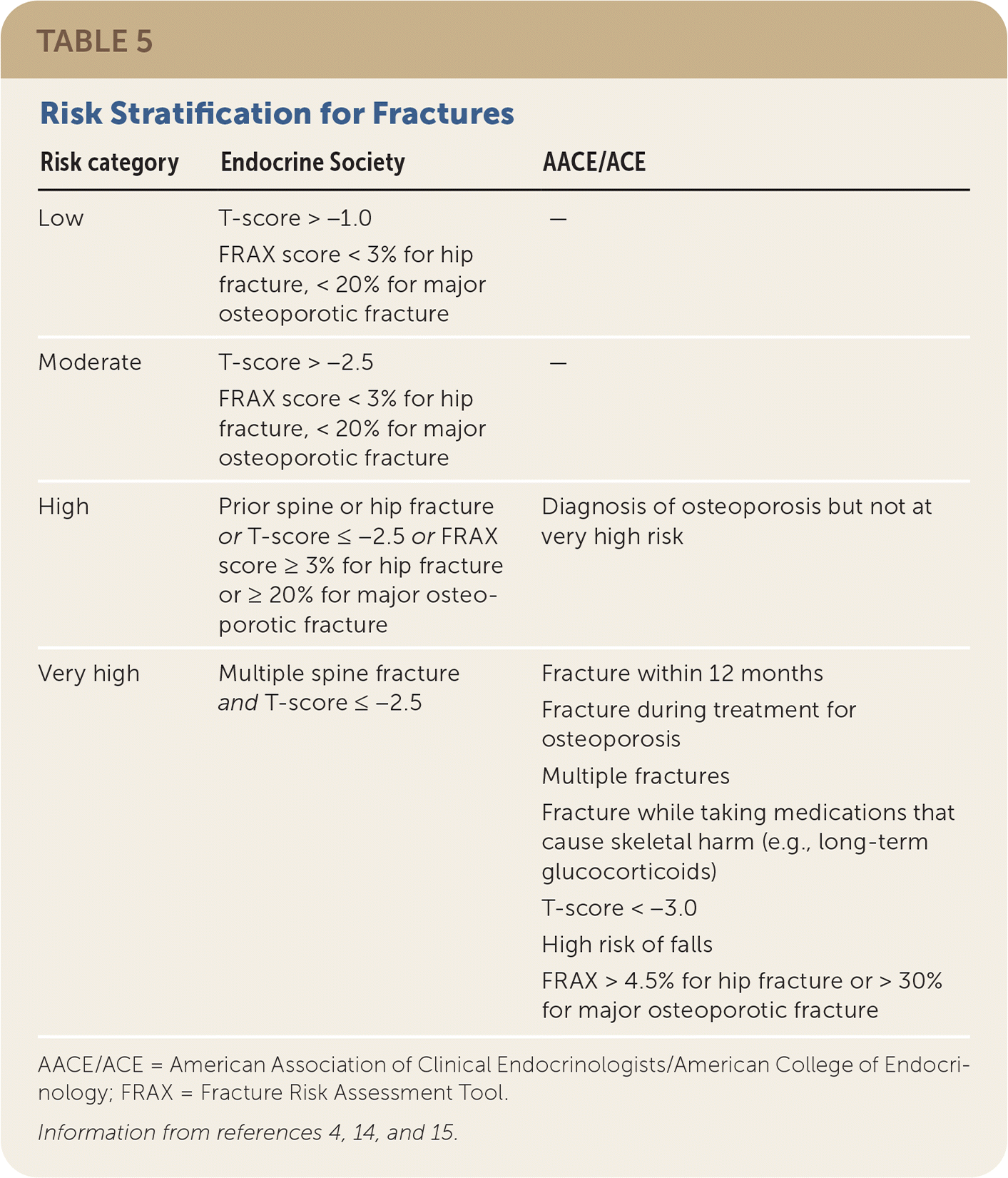
| Risk category | Endocrine Society | AACE/ACE |
|---|---|---|
| Low | T-score > −1.0 FRAX score < 3% for hip fracture, < 20% for major osteoporotic fracture | — |
| Moderate | T-score > −2.5 FRAX score < 3% for hip fracture, < 20% for major osteoporotic fracture | — |
| High | Prior spine or hip fracture or T-score ≤ −2.5 or FRAX score ≥ 3% for hip fracture or ≥ 20% for major osteoporotic fracture | Diagnosis of osteoporosis but not at very high risk |
| Very high | Multiple spine fracture and T-score ≤ −2.5 | Fracture within 12 months Fracture during treatment for osteoporosis Multiple fractures Fracture while taking medications that cause skeletal harm (e.g., long-term glucocorticoids) T-score < −3.0 High risk of falls FRAX > 4.5% for hip fracture or > 30% for major osteoporotic fracture |
What Are the Preferred First-line Osteoporosis Treatment Options, and What Are Their Risks and Benefits?
A patient’s fracture risk determines initial therapy. Patients at high risk of fracture should receive antiresorptive therapy with proven hip fracture reduction. This includes bisphosphonates, specifically alendronate, risedronate, or zoledronic acid, and denosumab. Patients at very high risk of fracture, especially those with a recent fracture, should consider therapy with anabolic agents.
EVIDENCE SUMMARY
Recent meta-analyses have shown that most medications used for osteoporosis significantly decrease vertebral fracture risk in women who are postmenopausal (Table 64,5,13–16). Zoledronic acid (intravenous bisphosphonate; Reclast) and denosumab (RANK ligand inhibitor) showed a significantly lower risk of vertebral fractures compared with oral bisphosphonates, with no difference in hip fracture risk.13,16 Nonvertebral fracture risk was decreased by bisphosphonates, denosumab, parathyroid hormone analogues, and romosozumab (Evenity; sclerostin inhibitor). Agents consistently shown to significantly decrease hip fracture risk were bisphosphonates (alendronate [Fosamax], risedronate [Actonel], zoledronic acid) and denosumab.13,16 Based on their efficacy in hip fracture reduction, these antiresorptive agents are first-line therapies for most patients at high fracture risk.4,14 The American College of Physicians conducted a systematic review that similarly confirmed the effectiveness of bisphosphonates and denosumab in reducing hip and vertebral fractures, and it recommends bisphosphonates as first-line therapy and RANK ligand inhibitors as second-line therapy.17,18
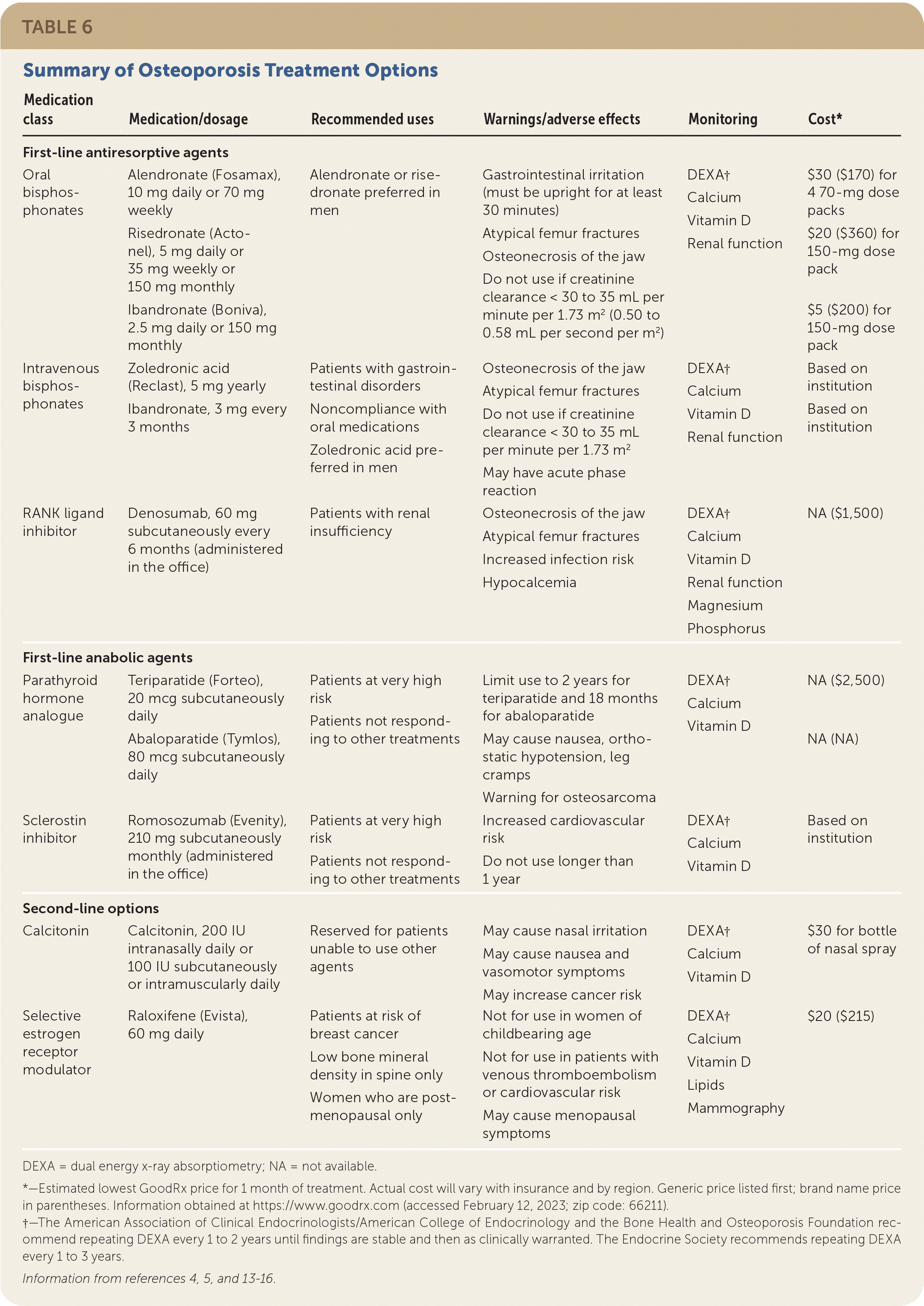
| Medication class | Medication/dosage | Recommended uses | Warnings/adverse effects | Monitoring | Cost* |
|---|---|---|---|---|---|
| First-line antiresorptive agents | |||||
| Oral bisphosphonates | Alendronate (Fosamax), 10 mg daily or 70 mg weekly Risedronate (Actonel), 5 mg daily or 35 mg weekly or 150 mg monthly Ibandronate (Boniva), 2.5 mg daily or 150 mg monthly | Alendronate or risedronate preferred in men | Gastrointestinal irritation (must be upright for at least 30 minutes) Atypical femur fractures Osteonecrosis of the jaw Do not use if creatinine clearance < 30 to 35 mL per minute per 1.73 m2 (0.50 to 0.58 mL per second per m2) | DEXA† Calcium Vitamin D Renal function | $30 ($170) for 4 70-mg dose packs $20 ($360) for 150-mg dose pack $5 ($200) for 150-mg dose pack |
| Intravenous bisphosphonates | Zoledronic acid (Reclast), 5 mg yearly Ibandronate, 3 mg every 3 months | Patients with gastrointestinal disorders Noncompliance with oral medications Zoledronic acid preferred in men | Osteonecrosis of the jaw Atypical femur fractures Do not use if creatinine clearance < 30 to 35 mL per minute per 1.73 m2 May have acute phase reaction | DEXA† Calcium Vitamin D Renal function | Based on institution Based on institution |
| RANK ligand inhibitor | Denosumab, 60 mg subcutaneously every 6 months (administered in the office) | Patients with renal insufficiency | Osteonecrosis of the jaw Atypical femur fractures Increased infection risk Hypocalcemia | DEXA† Calcium Vitamin D Renal function Magnesium Phosphorus | NA ($1,500) |
| First-line anabolic agents | |||||
| Parathyroid hormone analogue | Teriparatide (Forteo), 20 mcg subcutaneously daily Abaloparatide (Tymlos), 80 mcg subcutaneously daily | Patients at very high risk Patients not responding to other treatments | Limit use to 2 years for teriparatide and 18 months for abaloparatide May cause nausea, orthostatic hypotension, leg cramps Warning for osteosarcoma | DEXA† Calcium Vitamin D | NA ($2,500) NA (NA) |
| Sclerostin inhibitor | Romosozumab (Evenity), 210 mg subcutaneously monthly (administered in the office) | Patients at very high risk Patients not responding to other treatments | Increased cardiovascular risk Do not use longer than 1 year | DEXA† Calcium Vitamin D | Based on institution |
| Second-line options | |||||
| Calcitonin | Calcitonin, 200 IU intranasally daily or 100 IU subcutaneously or intramuscularly daily | Reserved for patients unable to use other agents | May cause nasal irritation May cause nausea and vasomotor symptoms May increase cancer risk | DEXA† Calcium Vitamin D | $30 for bottle of nasal spray |
| Selective estrogen receptor modulator | Raloxifene (Evista), 60 mg daily | Patients at risk of breast cancer Low bone mineral density in spine only Women who are postmenopausal only | Not for use in women of childbearing age Not for use in patients with venous thromboembolism or cardiovascular risk May cause menopausal symptoms | DEXA† Calcium Vitamin D Lipids Mammography | $20 ($215) |
Anabolic agents promote rapid bone growth for patients with low T-scores (less than −3.0) and may provide benefits to patients categorized as having a very high risk of fracture, specifically vertebral fractures. Recent meta-analyses suggest parathyroid hormone analogues (teriparatide [Forteo], abaloparatide [Tymlos]) decreased vertebral fractures significantly compared with oral bisphosphonates.13,16 Romosozumab is the newest medication and provides anabolic and antiresorptive properties. In addition to significant reductions in vertebral and clinical fractures, one meta-analysis suggested romosozumab significantly decreased hip fracture risk by 56% compared with placebo.13 In a head-to-head study compared with alendronate, romosozumab resulted in 48% fewer vertebral fractures, 27% fewer clinical fractures, and 38% fewer hip fractures in patients with a history of vertebral fracture.19 Additional studies are warranted to confirm this benefit; however, updates to treatment guidelines recommend romosozumab for patients at very high risk of fracture.4,15
Convenience and adverse effects are important considerations. Oral bisphosphonates can be administered weekly or monthly but require patients to take them on an empty stomach and stay upright for at least 30 minutes. Intravenous bisphosphonates may be preferred for patients at risk of esophageal erosion due to comorbid conditions or an inability to follow administration recommendations. Denosumab is administered every six months but is more expensive. Both antiresorptive classes carry the risk of atypical femur fractures and osteonecrosis of the jaw with prolonged therapy. Parathyroid hormone analogues are administered subcutaneously daily, whereas romosozumab is administered in-office monthly. In one clinical trial with romosozumab, a small increase in cardiac ischemic events was found in patients with cardiovascular risk factors (0.8% vs. 0.3% with alendronate).19 Patients with or at risk of cardiovascular disease should consider alternative therapy. Figure 1 is an algorithm for the treatment of osteoporosis.4,5,14,15
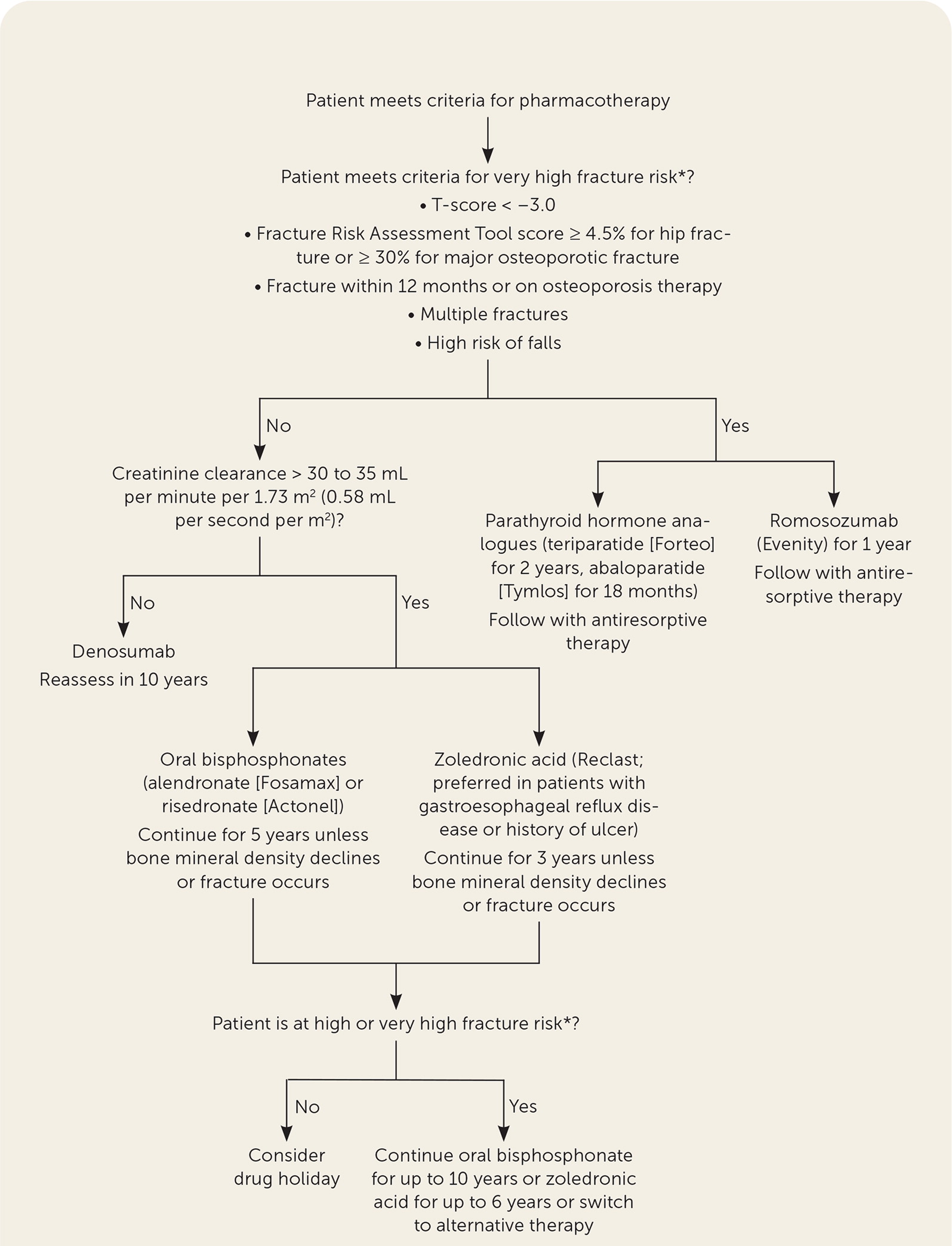
How Long Should Patients Receive Treatment for Osteoporosis?
Patients receiving oral or intravenous bisphosphonates should be reassessed after five years and three years of therapy, respectively, to determine if a drug holiday is appropriate. Patients at high fracture risk may continue oral therapy for up to 10 years or up to six years for intravenous therapy. Denosumab therapy should be reevaluated at five and 10 years. Teriparatide, abaloparatide, and romosozumab should be continued for two years, 18 months, and one year, respectively, followed by the usual course of antiresorptive therapy.
EVIDENCE SUMMARY
Bisphosphonate therapy is associated with a risk of atypical femur fractures, which increases with a prolonged duration of therapy. A prospective cohort study in 2020 found that in women receiving bisphosphonates, atypical femur fractures occurred at a significantly lower rate of 1.74 per 10,000 person-years compared with the inherent risk of hip fractures in this population (58.9 per 10,000 person-years).20 However, the risk of atypical femur fracture increased with prolonged therapy. Compared with bisphosphonate therapy for less than three months, the multivariate-adjusted hazard ratio of atypical femur fracture was significantly greater in women receiving bisphosphonate therapy for three to five years (8.86; 95% CI, 2.79 to 28.2), five to eight years (19.88; 95% CI, 6.32 to 62.49), and more than eight years (43.51; 95% CI, 13.7 to 138.15).20 Other risk factors associated with atypical femur fracture included Asian race (as reported by patients), decreasing height, increasing weight, age 65 to 74 years, and more than one year of glucocorticoid use.20 Overall, the benefit of osteoporotic fracture reduction greatly outweighed the risk of atypical femur fracture through five years of therapy.20 Discontinuation of bisphosphonates can decrease the risk of atypical femur fracture while maintaining fracture risk benefit. Previous studies have shown that BMD and risk of fracture are unchanged for several years following discontinuation after five years of oral therapy and three years of intravenous therapy with zoledronic acid 21,22; however, a systematic review found some evidence that more prolonged therapy with alendronate may decrease clinical fractures in women with a lower BMD.23 The 2020 cohort study showed that when bisphosphonate therapy was discontinued, the risk of atypical femur fracture decreased by 48% after three to 15 months and by 74% to 78% after that.20 This study supports previous findings by a retrospective study indicating that the odds ratio for atypical femur fracture after four to five years of bisphosphonate use declined by 70% every year after discontinuation.24
Several organizations recommend that patients consider a drug holiday after five years of oral therapy or three years of intravenous therapy.4,14,25 A drug holiday is defined as a period of up to five years when patients are receiving no therapy.4,14,25 For patients who are at high risk of fracture, oral bisphosphonates can be considered for up to 10 years, intravenous bisphosphonates for up to six years, or alternative therapy.4,14,25 Denosumab therapy has also been associated with atypical femur fracture; however, BMD declines, and fracture risk increases following therapy discontinuation. Patients receiving denosumab should not take a drug holiday.4,14 Patients should be reevaluated at five to 10 years of therapy to consider alternative therapy.
Anabolic agents have optimal durations to maximize effectiveness and decrease risks. Both parathyroid hormone analogues, teriparatide and abaloparatide, are approved for up to two years; however, clinical trials demonstrating improvements in BMD and fracture risk with abaloparatide lasted only 18 months.26 Romosozumab is approved for one year of therapy because prolonged therapy provided limited additional benefit. Because of the rapid loss of BMD after discontinuation of anabolic agents, antiresorptive therapy should be initiated following therapy completion.4,14,15
This article updates previous articles on this topic by Jeremiah, et al.27; Sweet, et al.28; South-Paul29; and Ullom-Minnich.30
Data Sources: A search of PubMed and Ovid was completed using the key terms osteoporosis, diagnosis, and treatment. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. A search of Essential Evidence Plus for osteoporosis in February 2022 was also completed. Search dates: January 2022, May 2022, and October 5, 2022.
