
Am Fam Physician. 2023;107(3):316-317
Clinical Question
Are amitriptyline, duloxetine (Cymbalta), and pregabalin (Lyrica) effective in decreasing pain in adults with diabetic peripheral neuropathy?
Bottom Line
Adults with painful diabetic peripheral neuropathy had similar degrees of improvement with monotherapy using amitriptyline, duloxetine, and pregabalin. There was even greater improvement with subsequent combination therapy regardless of initial choice of medication. (Level of Evidence = 1b)
Synopsis
The researchers enrolled 130 adults with diabetes mellitus and pain associated with distal symmetric polyneuropathy for at least three months. In this crossover trial, the participants were randomly assigned to three 16-week pathways separated by a two-week washout period: oral amitriptyline supplemented with pregabalin, pregabalin supplemented with amitriptyline, and duloxetine supplemented with pregabalin. Each pathway started with a two-week period in which doses were titrated to the maximum tolerated dose. This was followed by six weeks of maintenance monotherapy. At the end of six weeks, those with a pain level of 3 or less out of 10 were classified as responders and remained on monotherapy for 10 weeks. Nonresponders received the second drug for 10 weeks. During the subsequent 10 weeks, the researchers titrated medication doses to maintain pain levels at 3 or less out of 10. At the end of 16 weeks, the researchers stopped all study drugs for a two-week washout period, and the participants started the next drug combination.
Most (84%) of the participants had type 2 diabetes and were White (94%). Although 130 started the first pathway, only 97 and 84 began a second and third pathway, respectively. At the end of six weeks of monotherapy, the proportion of responders was similar for amitriptyline (37%), duloxetine (32%), and pregabalin (34%). At the end of 16 weeks, the proportion of responders was similar for oral amitriptyline supplemented with pregabalin (48%), pregabalin supplemented with amitriptyline (47%), and duloxetine supplemented with pregabalin (43%). The authors found that at the end of each evaluation period, the participants had similar degrees of improvement in pain regardless of agent. Most adverse events were mild and similar across all three pathways, with the three exceptions summarized in the accompanying table.
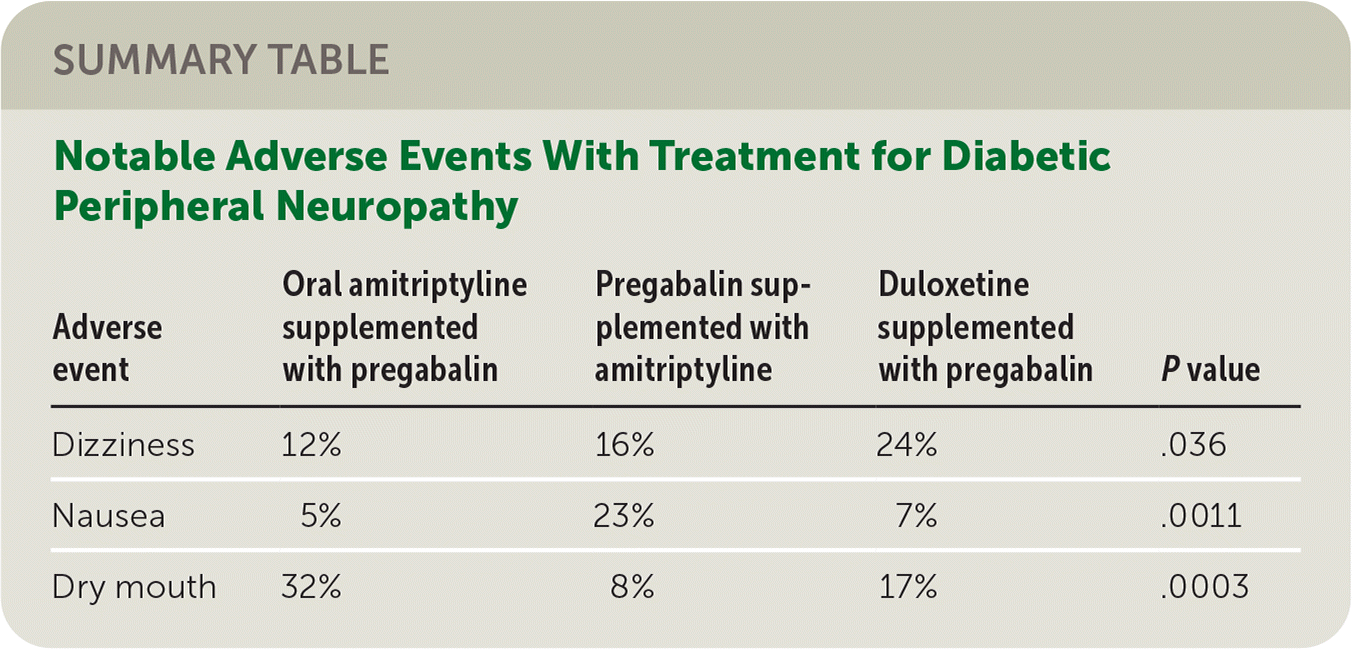
| Adverse event | Oral amitriptyline supplemented with pregabalin | Pregabalin supplemented with amitriptyline | Duloxetine supplemented with pregabalin | Pvalue |
|---|---|---|---|---|
| Dizziness | 12% | 16% | 24% | .036 |
| Nausea | 5% | 23% | 7% | .0011 |
| Dry mouth | 32% | 8% | 17% | .0003 |
Study design: Crossover trial (randomized)
Funding source: Government
Allocation: Concealed
Setting: Outpatient (any)
Reference: Tesfaye S, Sloan G, Petrie J, et al.; OPTION-DM Trial Group. Comparison of amitriptyline supplemented with pregabalin, pregabalin supplemented with amitriptyline, and duloxetine supplemented with pregabalin for the treatment of diabetic peripheral neuropathic pain (OPTION-DM): a multi-centre, double-blind, randomised crossover trial [published correction appears in Lancet. 2022;400(10355):810]. Lancet. 2022;400(10353):680-690.
