
Am Fam Physician. 2023;108(1):78-83
Patient information: See related handout on mpox.
Published online June 12, 2023.
Author disclosure: No relevant financial relationships.
Mpox (formerly monkeypox) is a DNA virus of the Orthopoxvirus genus, similar to smallpox. Although mpox was endemic to the Democratic Republic of the Congo and parts of Africa, increasing numbers of cases were reported worldwide in 2022. More than 30,000 cases have been reported in the United States, and worldwide 98% of cases are found in men who have sex with men. Transmission is primarily through contact with skin lesions. The rash of mpox is often vesiculopustular and may be localized to the anogenital region or distributed on the face, trunk, limbs, palms, and soles. Two vaccines are available for pre- or postexposure prophylaxis. Jynneos (smallpox and mpox vaccine, live, nonreplicating) is a live, attenuated vaccine that is safe for patients who are immunocompromised. ACAM2000 (smallpox [vaccinia] vaccine, live) is a live vaccinia virus vaccine that should be given only to immunocompetent, nonpregnant people and should be avoided in those with skin conditions such as atopic dermatitis. For most people infected with mpox, the disease is mild and self-limiting. Antiviral treatments such as tecovirimat, cidofovir, or brincidofovir may be considered for use in individuals who have or are at high risk of severe disease. Possible complications of mpox include anogenital pain, bacterial superinfections of skin lesions, dehydration secondary to oral lesions, encephalitis, keratitis, and respiratory distress. To date, 38 deaths have been reported in the United States.
Mpox (formerly known as monkeypox) is a DNA virus of the Orthopoxvirus genus, the same genus as smallpox. It was first isolated in monkeys in 1958; the first human case was identified in the Democratic Republic of the Congo (previously Zaire) in 1970.1 This article provides a summary and review of the best available patient-oriented evidence for mpox. Additional information about mpox is available from the American Academy of Family Physicians.2
Epidemiology
In addition to monkeys and apes, dogs, hedgehogs, and some rodent species (including squirrels and prairie dogs) can be infected with mpox.3
Before 2022, most cases of mpox occurred in the Democratic Republic of the Congo.4 The first reported cases of mpox outside Africa occurred in the Midwest United States in 2003.4,5 The outbreak was traced back to prairie dog pets that were infected by exotic animals imported from Ghana.4,5
Starting in 2022, increasing numbers of cases of mpox have been reported outside the African continent. A case series of 528 infections diagnosed between April 27 and June 24, 2022, and involving 16 countries found that 98% of people infected were gay or bisexual men and that 41% of those infected were coinfected with HIV. Among those infected, 29% had a concomitant sexually transmitted infection. Hospitalization was required for 70 patients (13%) for pain control, soft tissue superinfection, pharyngitis, and infection control. No deaths were reported.6
As of June 7, 2023, a total of 30,468 cases of mpox had been confirmed in the United States.7 World Health Organization data indicate a global total of 87,929 cases, with 146 deaths as of June 6, 2023.8
Prevention
Transmission is primarily through contact with skin lesions. Skin-to-skin contact should be avoided with those suspected to have mpox. Gloves should be used when managing the laundry of people thought to be infected.9,10
In addition to skin lesions, the virus has also been detected in samples taken from the anus, throat, blood, urine, and semen of individuals infected with mpox.11
For health care workers caring for patients with suspected mpox, wearing personal protective equipment with gown and gloves is recommended to prevent transmission. Because risk of spread by the respiratory route can occur, eye protection and N95 respirators are also recommended.9,12
In the hospital, patients do not necessarily need a negative pressure room. Patients should have their own room with a private bathroom. Lesions should be covered with protective clothing or gauze when possible.9
Individuals should avoid sex (anal, oral, or vaginal) and intimate contact with people who have mpox.13 Individuals can reduce their risk of being exposed to mpox by limiting the number of sex partners, avoiding sex clubs and sex parties, and limiting intimate contact in high-risk settings. Use of condoms and gloves may help prevent transmission, as can other barriers to skin-to-skin contact.13
Those recovered from mpox and their sex partners should use barrier protection during intercourse for at least 12 weeks after resolution of symptoms to prevent spread to others.14
A survey from the Centers for Disease Control and Prevention (CDC) shows that communication about the monkeypox virus leads to changes in sexual behaviors among men who have sex with men.15
VACCINATION
Two vaccines are currently licensed by the U.S. Food and Drug Administration (FDA) for use against mpox.
Jynneos (smallpox and mpox vaccine, live, non-replicating) is a live, attenuated vaccine that is safe for immunocompromised patients and comprises two doses, four weeks apart. Incidence of mpox was 14 times higher in those who were unvaccinated compared with those who received at least one dose of the Jynneos vaccine.16,17 Redness, swelling, and pain at the site of the injection are common. In trials, serious adverse events occurred at a rate of 1.5% in those given Jynneos compared with 1.1% in the placebo group among patients who were vaccinia vaccine naive.18 Acute cardiac adverse events of special interest occurred in 1.3% of those given Jynneos compared with 0.2% in those given placebo.18 Events included asymptomatic troponin I elevation, electrocardiogram abnormalities, and palpitations; none were considered serious.18 A 0.5-mL dose of Jynneos is given subcutaneously to patients younger than 18 years, whereas those 18 years and older may be given a smaller 0.1-mL dose intradermally, according to CDC guidance.19,20
ACAM2000 (smallpox [vaccinia] vaccine, live) is a live vaccinia virus vaccine that should be given only to immunocompetent, nonpregnant people and should be avoided in those with skin conditions such as atopic dermatitis. It should not be used in people who have HIV or AIDS.21 This vaccine comprises one percutaneous dose via a multiple puncture technique with a bifurcated needle.
It is possible to transmit the ACAM2000 vaccine to close contacts. Precautions should be taken to avoid accidental inoculation (e.g., covering the vaccination site and the lesion that the vaccination produces with a gauze bandage and washing hands with soap and water or using an alcohol-based hand sanitizer after contact with the site or after covering with bandaging, clothing, or linens (e.g., sheets, pillowcases, towels). ACAM2000 carries an FDA boxed warning for possible myocarditis and pericarditis (5.7 per 1,000 vaccinees; 95% CI, 1.9 to 13.3), and rare instances of encephalitis (1.4 to 24.7 per million) and generalized vaccinia (58.9 to 74.2 per million in the 2002 to 2005 administration of a previous iteration of live vaccinia vaccine) have occurred.22 ACAM2000 may be used in patients one year or older.21
PRE-EXPOSURE AND POSTEXPOSURE PROPHYLAXIS
Either vaccine can be used for pre-exposure prophylaxis (PrEP) or postexposure prophylaxis (PEP).
PEP involves vaccination after known or presumed exposure to mpox, ideally within four days of the patient's exposure. PEP may be given up to 14 days after exposure.23 PEP may also be given to those with risk factors or experiences that increase their likelihood of being exposed to mpox.23
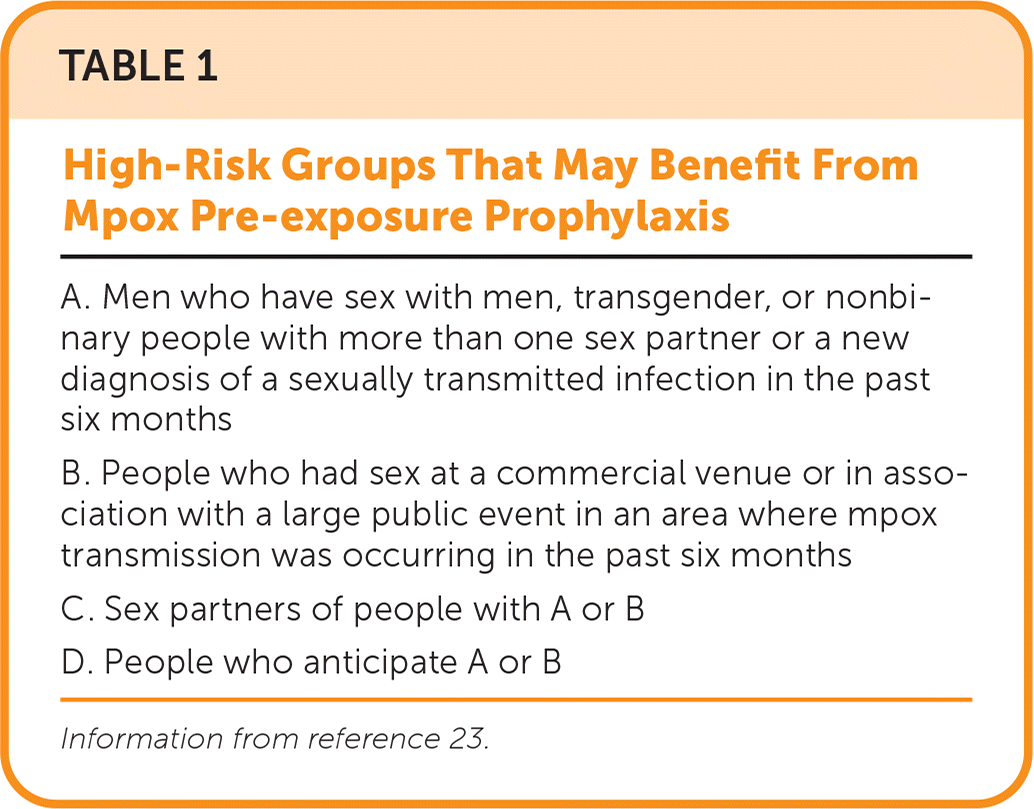
PrEP involves administering the vaccine to those at high risk of exposure (Table 1).23
At this time, those who suspect mpox exposure should contact their state health department to determine whether they qualify for PEP.24
Patients who are vaccinated with PEP should receive either the Jynneos or the ACAM2000 vaccine within four days to prevent infection or up to 14 days from exposure to minimize symptoms.14
|
Diagnosis
Mpox should be suspected in those with a characteristic rash (i.e., often pustular and may be present in the anogenital region and/or face, trunk, and extremities) and risk factors for infection (e.g., men who have sex with men, contact with someone suspected of having mpox).6,9
The differential diagnosis includes herpes simplex virus, gonorrhea, syphilis, varicella zoster virus, other pox viruses (e.g., molluscum contagiosum), Behçet syndrome, and recurrent aphthous stomatitis. However, the presence of one of these conditions does not exclude mpox because these may copresent.9,25
SIGNS AND SYMPTOMS
The most prominent feature of mpox is a rash, which is often pustular and may be present in the anogenital region and/or face, trunk, and extremities (eFigure A and eFigure B). In the 2022 outbreak, anogenital lesions were present in 73% of detected cases.6 The patient's history may include contact with an infected individual or a high-risk social network, followed by a viral prodrome that involves fevers, myalgias, fatigue, and lymphadenopathy.6
Mpox historically comprises an incubation period, prodromal period, development of rash, and resolution of rash.
Patients are asymptomatic during the incubation period, which may last from five to 21 days.25 If present, the prodrome most commonly includes fever (62%), malaise (41%), lymphadenopathy (56%), headache (27%), and myalgias (31%).6 Rectal pain, rectal bleeding, and tenesmus were also noted.6 In the 2022 outbreak, many patients had no prodromal symptoms.6
The rash of mpox can vary and is often vesiculopustular. Lesions may be localized to the anogenital region or distributed on the face, palms, and soles.25 Some patients may present with only proctitis.6 The typical course of the rash involves macules (one to two days), papules (one to two days), vesicles (one to two days), pustules (five to seven days), and scabs (seven to 14 days).9
The patient is no longer considered contagious after all scabs have fallen off and the underlying skin is intact.9


DIAGNOSTIC TESTING
Lesions can be tested for mpox with polymerase chain reaction testing. Two specimens from each of two or three separate lesions should be collected with nylon, polyester, or Dacron swabs. Specimens should be stored in dry, sterile containers with tight-fitting caps and kept refrigerated or frozen until testing.26
Polymerase chain reaction testing is highly sensitive and specific for monkeypox virus.27
In addition to testing for mpox, it may be prudent to cotest for other sexually transmitted infections, especially HIV. Certain mpox treatments require renal dosing or close monitoring of renal function, so a baseline metabolic panel can be helpful when considering treatment.28
The CDC recommends contacting the state health department or the CDC emergency operations center (770-488-7100) for further information on testing when mpox is suspected.24
Treatment
DRUG THERAPY
For most individuals infected with mpox, the disease is mild and self-limiting.29
Monkeypox and smallpox viruses are genetically similar, and antiviral medications and vaccines developed for smallpox may be used with mpox. However, none of the available antivirals have been evaluated in a clinical trial of patients with mpox.29
In consultation with a physician, local health department, or the CDC, antiviral treatment should be considered for individuals who have or are at high risk of severe disease (Table 2).29
Tecovirimat (Tpoxx) has been the most used antiviral medication during the current outbreak. Tecovirimat is an FDA-approved treatment for smallpox and is available to treat mpox through expanded access for an investigational new drug protocol.28
Animal studies have demonstrated that tecovirimat is effective in treating disease caused by Orthopoxviruses.30 One small case series suggests that tecovirimat may shorten the duration of illness and viral shedding in human mpox.31
Clinical trials in people show that the drug is safe and causes only minor adverse effects, which include headache, nausea, abdominal pain, and vomiting. Use should be monitored in patients with renal impairment.28
Tecovirimat is available in oral and intravenous formulations. If used orally, it should be taken 30 minutes after a full meal of higher fat content to improve bioavailability.28
In addition to tecovirimat, cidofovir and brincidofovir (Tembexa) are other antivirals that have shown effectiveness against Orthopoxviruses.29,32,33
CNJ-016 (vaccinia immune globulin) is approved by the FDA for treating vaccinia vaccination complications such as eczema vaccinatum and progressive and severe generalized vaccinia.34 Similar to several of the other treatments, an expanded access for an investigational new drug protocol authorizes its use in Orthopoxvirus outbreaks, including mpox. CNJ-016 can also be considered for use in patients exposed to mpox when smallpox vaccination is contraindicated.29
The mainstay of clinical management for a typical mpox infection involves supportive care, including fluid maintenance, hemodynamic support, management of complications (e.g., auto-inoculation to the eyes, mouth, and genital area), and pain management.35

| Severe disease |
| Encephalitis |
| Hemorrhagic disease |
| Hospitalization |
| Large number of lesions that are confluent |
| Myocarditis |
| Sepsis |
| At risk of severe disease |
| Active exfoliating skin conditions |
| Acne, severe |
| Atopic dermatitis |
| Burns |
| Diaper dermatitis, severe |
| Eczema |
| Herpes simplex virus |
| Impetigo |
| Keratosis follicularis |
| Varicella-zoster virus infection |
| Anogenital disease or accidental inoculation in eyes, mouth |
| Children, especially those younger than eight years |
| Complications of mpox |
| Pneumonia |
| Secondary bacterial skin infection |
| Severe dehydration, diarrhea, nausea, or vomiting |
| Immunocompromise |
| HIV infection or AIDS |
| Leukemia or lymphoma |
| Malignancy |
| Solid organ transplantation |
| Stem cell transplantation (< 24 months prior or with active graft-vs.-host disease) |
| Therapy with immunocompromising agents (e.g., alkylating agents, antimetabolites, corticosteroids, radiation, tumor necrosis factor inhibitors) |
| Pregnant or breastfeeding individuals |
REFERRAL, CONSULTATION, AND HOSPITALIZATION
In a case series involving 16 countries, hospitalization was most often used for pain control, soft tissue superinfection, and pharyngitis.6
The decision to hospitalize should be made on a case-by-case basis, considering the location and severity of the disease, the patient's comorbidities, and, potentially, the risk of spread.
Prognosis
During the current outbreak, cases of mpox have been mild, with 42 reported deaths in the United States at the time of this publication.36
Prognosis is determined by several factors, including location of disease, previous vaccinations, and comorbid conditions, including immunosuppression.
Two distinct genetic clades of mpox exist: clade I and clade II. The current global outbreak involves clade II, which typically causes self-limited disease.35
Conditions associated with a higher risk of poor outcomes are listed in Table 2.29
Possible complications include bacterial super-infections of skin lesions, dehydration secondary to oral lesions, encephalitis, keratitis, respiratory distress, and death.37
Editor's Note: Dr. Saguil is an assistant medical editor for AFP.
Data Sources: Literature sources searched include PubMed, Essential Evidence Plus, federal government websites (including the CDC and FDA), and websites associated with global health (such as the World Health Organization). Search terms included monkeypox, orthopoxvirus, smallpox, and derivatives of these terms. Search dates: July 2022 through April 2023.
