
Am Fam Physician. 2024;109(1):online
Author disclosure: No relevant financial relationships.
Key Points for Practice
• For HIV PrEP, options include daily or episodic emtricitabine/tenofovir disoproxil fumarate (Truvada) or daily emtricitabine/tenofovir alafenamide (Descovy) and injectable cabotegravir (Apretude) every four to eight weeks.
• Rapid treatment initiation after HIV diagnosis improves outcomes; starting within seven days is recommended in patients without opportunistic infections and within 14 days in patients with most opportunistic infections.
• Most treatment failures are due to missed doses, so simplifying treatment regimens can be effective for patients with and without virologic suppression.
From the AFP Editors
Four decades after the initial HIV case was reported, strategies for treating and preventing the disease continue to advance. In the United States, the HIV epidemic continues to disproportionately affect people who are Black or Hispanic, men who have sex with men, people who are transgender, and individuals who use illicit drugs. HIV is increasingly diagnosed in older adults, and one-fourth of HIV-positive people will be older than 65 years by 2030.
HIV Prevention
Condom use remains the foundation of HIV prevention. Preexposure prophylaxis (PrEP) is proven to reduce incidence and should be discussed with all sexually active adults and adolescents, especially cisgender men and trans-gender individuals who have sex with men, people with a recent sexually transmitted infection, and those with substance use disorder or who inject nonprescription drugs. PrEP can be started immediately with negative point-of-care HIV testing or negative testing within seven days. Regular laboratory testing is recommended for patients taking PrEP (Table 1).
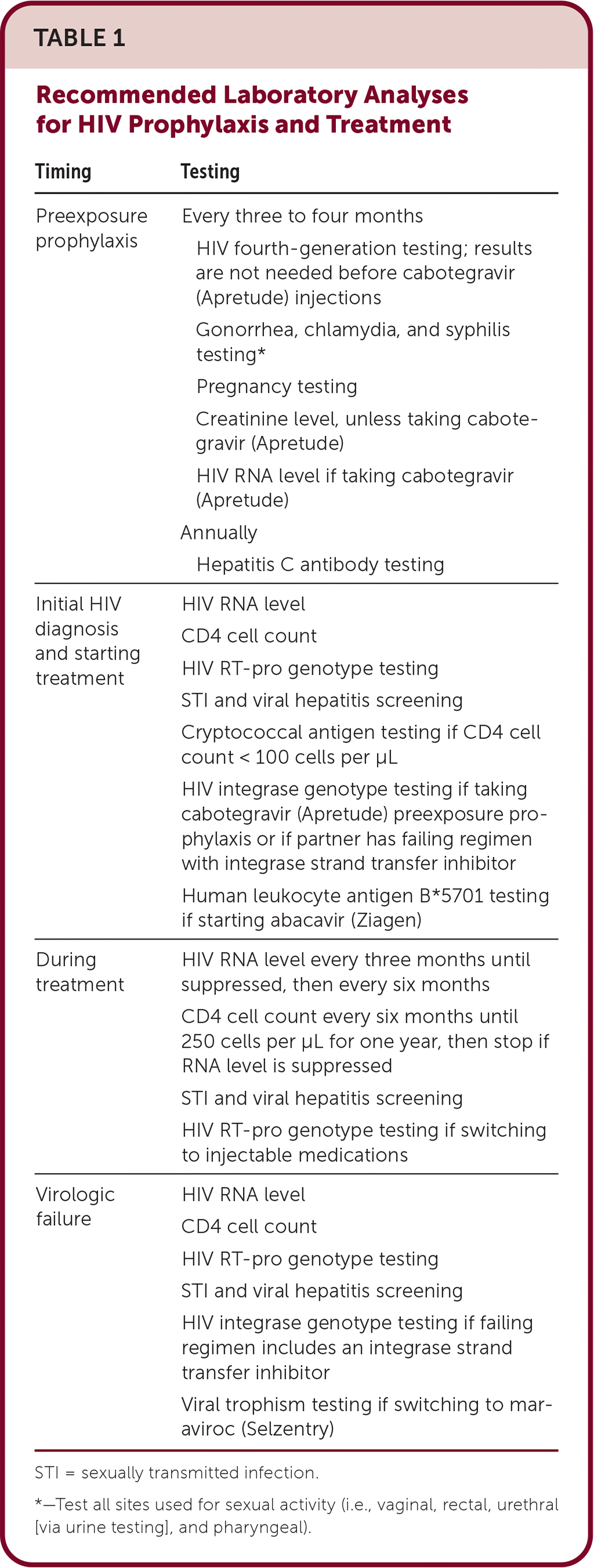
| Timing | Testing |
|---|---|
| Preexposure prophylaxis | Every three to four months HIV fourth-generation testing; results are not needed before cabotegravir (Apretude) injections Gonorrhea, chlamydia, and syphilis testing* Pregnancy testing Creatinine level, unless taking cabotegravir (Apretude) HIV RNA level if taking cabotegravir (Apretude) Annually Hepatitis C antibody testing |
| Initial HIV diagnosis and starting treatment | HIV RNA level CD4 cell count HIV RT-pro genotype testing STI and viral hepatitis screening Cryptococcal antigen testing if CD4 cell count < 100 cells per μL HIV integrase genotype testing if taking cabotegravir (Apretude) preexposure prophylaxis or if partner has failing regimen with integrase strand transfer inhibitor Human leukocyte antigen B*5701 testing if starting abacavir (Ziagen) |
| During treatment | HIV RNA level every three months until suppressed, then every six months CD4 cell count every six months until 250 cells per μL for one year, then stop if RNA level is suppressed STI and viral hepatitis screening HIV RT-pro genotype testing if switching to injectable medications |
| Virologic failure | HIV RNA level CD4 cell count HIV RT-pro genotype testing STI and viral hepatitis screening HIV integrase genotype testing if failing regimen includes an integrase strand transfer inhibitor Viral trophism testing if switching to maraviroc (Selzentry) |
Multiple PrEP dosing regimens are effective. Emtricitabine/tenofovir disoproxil fumarate (Truvada) is effective when taken daily and for on-demand episodic use in those who do not use injection drugs, with a double dose two to 24 hours before sexual activity and single doses daily until 48 hours after sexual activity. Emtricitabine/tenofovir alafenamide (Descovy) is taken daily and is safer for use in chronic kidney disease and osteopenia but leads to more weight gain. Long-acting injectable cabotegravir (Apretude) is also effective; injections should start every four weeks and can be extended to eight weeks apart. Oral PrEP regimens are recommended after cabotegravir (Apretude) is discontinued.
Postexposure prophylaxis with 28 days of a three-drug regimen of bictegravir/emtricitabine/tenofovir alafenamide (Biktarvy); or dolutegravir (Tivicay) plus emtricitabine/tenofovir disoproxil fumarate (Truvada) or emtricitabine/tenofovir alafenamide (Descovy) or lamivudine/tenofovir disoproxil fumarate (Temixys) is recommended within 72 hours of exposure to blood or genital secretions from a person who is suspected to be HIV positive. For transgender individuals who have sex with men who are at high risk, adding a single 200-mg dose of doxycycline after intercourse without a condom should be considered to prevent gonorrhea, chlamydia, and syphilis.
Initiation of Antiretroviral Therapy
TIMING
Rapid initiation of treatment after diagnosis of HIV improves life expectancy and reduces HIV transmission to others. Initiation within seven days of diagnosis is recommended for rapid viral suppression. Barriers such as transportation, housing instability, racism, cost, and stigma should be addressed through case management and peer navigation.
If there is evidence of an opportunistic infection at the time of diagnosis, antiretroviral therapy can usually be started within two weeks of starting treatment of the infection. Patients with active tuberculosis can start antiretroviral therapy within two weeks of starting antibacterial treatment. When cryptococcal meningitis is diagnosed with HIV, antiretroviral therapy can be started two to four weeks after starting antifungal therapy. HIV treatment can be started immediately with cancer therapy.
MEDICATIONS
Initial regimens for HIV should include an integrase strand transfer inhibitor: dolutegravir (Tivicay) plus a tenofovir combination or bictegravir/emtricitabine/tenofovir alafenamide (Biktarvy; Table 2).
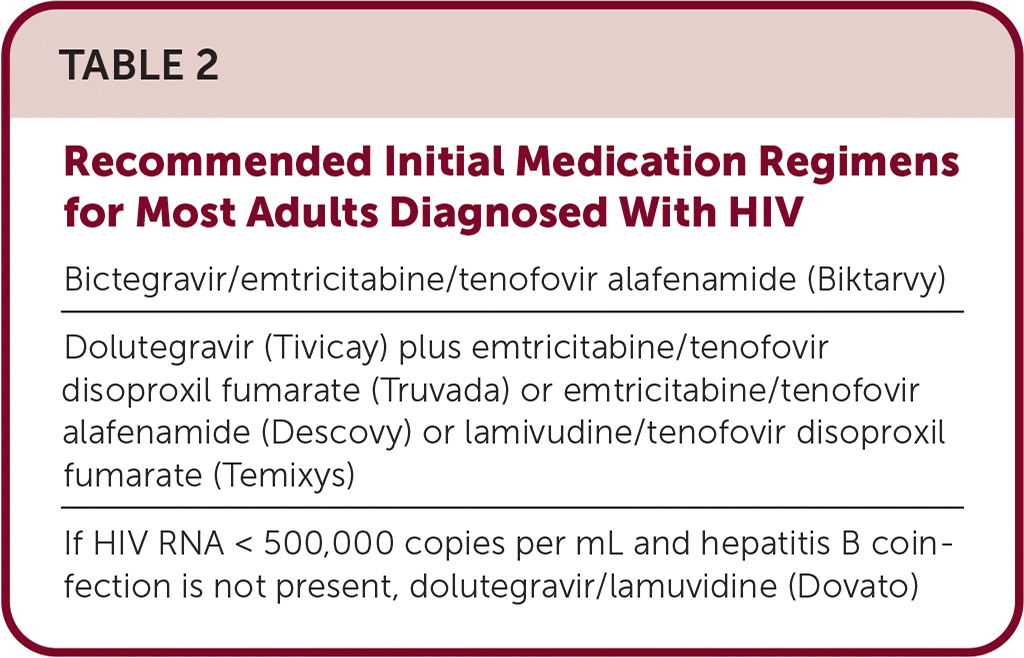
| Bictegravir/emtricitabine/tenofovir alafenamide (Biktarvy) |
| Dolutegravir (Tivicay) plus emtricitabine/tenofovir disoproxil fumarate (Truvada) or emtricitabine/tenofovir alafenamide (Descovy) or lamivudine/tenofovir disoproxil fumarate (Temixys) |
| If HIV RNA < 500,000 copies per mL and hepatitis B coinfection is not present, dolutegravir/lamuvidine (Dovato) |
If HIV is diagnosed in patients taking PrEP, treatment varies based on PrEP medication. If tenofovir was used for PrEP, a regimen with dolutegravir (Tivicay) plus a tenofovir combination is recommended (Table 2). Antiretroviral therapy resistance testing should be performed when medications are started. If cabotegravir (Apretude) was used for PrEP, wait for integrase strand transfer inhibitor genotyping or start darunavir (Prezista), tenofovir, and emtricitabine or lamivudine.
For HIV diagnosed during pregnancy, dolutegravir (Tivicay), emtricitabine/tenofovir alafenamide (Descovy), and lamivudine are the primary medication regimen recommended. Tenofovir disoproxil fumarate is an alternative to tenofovir alafenamide.
MONITORING
Monitoring during treatment includes HIV RNA levels and CD4 cell counts until proven stable (Table 1).
Switching Antiretroviral Medications
Before switching medications, treatment history, medications and tolerance, reproductive plans, insurance coverage, and resistance testing results should be reviewed. More frequent laboratory monitoring is recommended after switching medications.
For patients with viral suppression and no drug resistance, other regimens are generally safe, including the two-drug regimens of dolutegravir/lamivudine (Dovato) or dolutegravir/rilpivirine (Juluca). Cabotegravir (Apretude) and rilpivirine (Edurant) injections are convenient but have up to a 2% risk of resistance that can complicate future treatment.
When virologic failure occurs with HIV RNA levels greater than 200 copies per mL, medication adherence and resistance testing results should be assessed. With negative resistance testing results, tools to improve adherence, including simpler regimens, can be offered.
Primary Care
Although weight gain is an adverse effect of several HIV medications, switching medications does not seem to reduce weight gain. HIV and its treatment increase cardiac risk, and at least annual evaluation is recommended. Routine age- and risk-based screening is recommended for people with HIV, including regular sexually transmitted infection screening at exposed mucosal sites and screening for cancers, tuberculosis, and viral hepatitis.
Substance use disorders disproportionately affect people with HIV, and medication treatment improves HIV treatment outcomes.
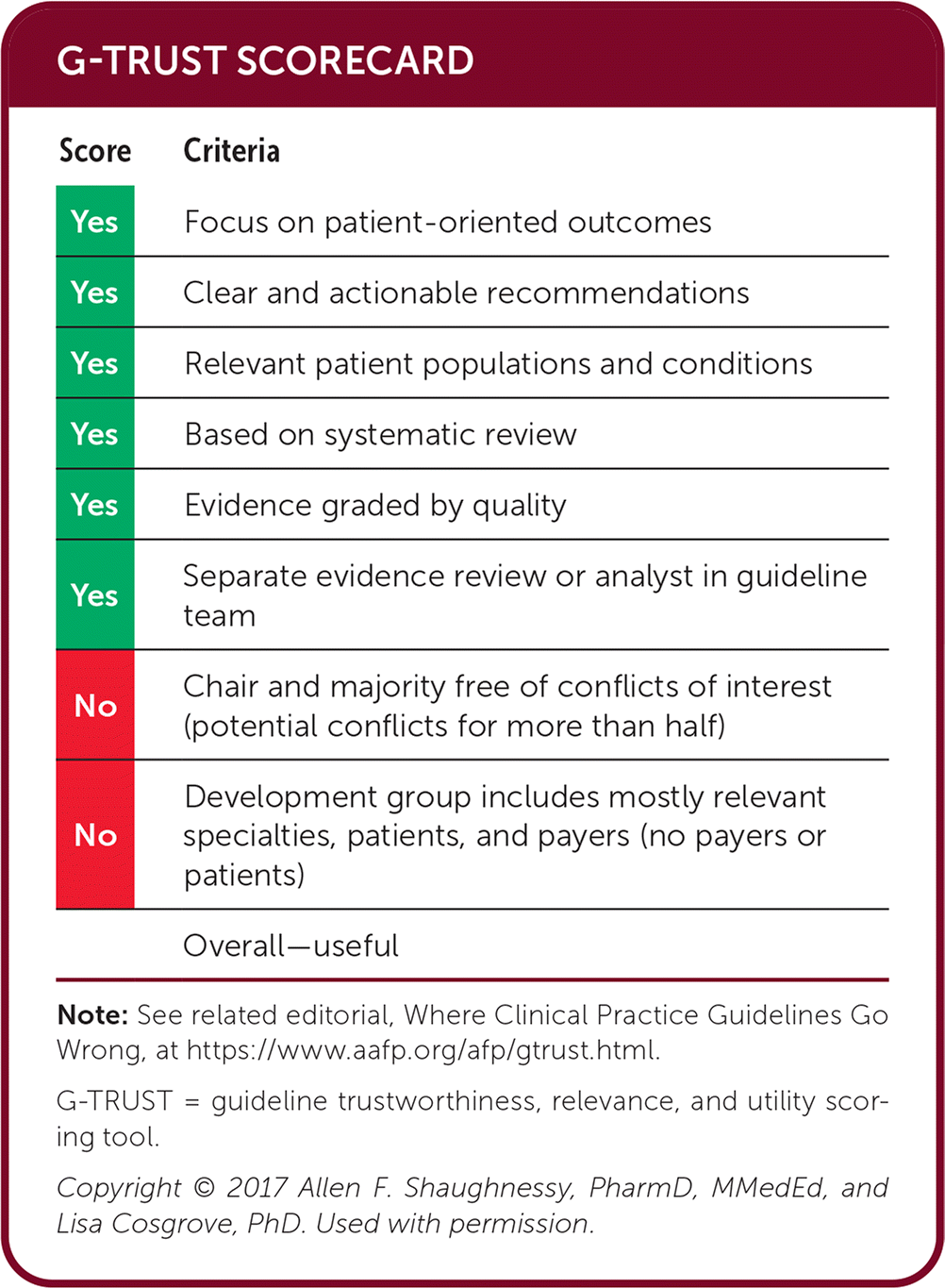
| Score | Criteria |
|---|---|
| Yes | Focus on patient-oriented outcomes |
| Yes | Clear and actionable recommendations |
| Yes | Relevant patient populations and conditions |
| Yes | Based on systematic review |
| Yes | Evidence graded by quality |
| Yes | Separate evidence review or analyst in guideline team |
| No | Chair and majority free of conflicts of interest (potential conflicts for more than half) |
| No | Development group includes mostly relevant specialties, patients, and payers (no payers or patients) |
| Overall—useful |
Editor's Note: This guideline contains an updated succinct evidence review of HIV prevention and treatment. Although most recommendations match guidance from the Centers for Disease Control and Prevention, these guidelines include more recent evidence.—Michael J. Arnold, MD, Contributing Editor
The views expressed are those of the author and do not necessarily reflect the official policy or position of the Uniformed Services University of the Health Sciences, Naval Undersea Medical Institute, U.S. Navy, U.S. Department of Defense, or U.S. government.
Guideline source: International Antiviral Society-USA Panel
Published source: Gandhi RT, Bedimo R, Hoy JF, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2022 recommendations of the International Antiviral Society-USA Panel. JAMA. 2023;329(1):63–84.
