
Am Fam Physician. 2024;110(2):199-200
Author disclosure: No relevant financial relationships.
Tirzepatide (Zepbound), also known as Mounjaro when used for the treatment of diabetes mellitus, acts on the glucagon-like peptide-1 (GLP-1) receptor and the gastric inhibitory polypeptide to regulate appetite and food intake. Zepbound is labeled as an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management in adults with a body mass index (BMI) greater than 30 kg per m2, or a BMI greater than 27 kg per m2 and at least one weight-related comorbidity (e.g., hypertension, dyslipidemia, type 2 diabetes, obstructive sleep apnea, cardiovascular disease).1
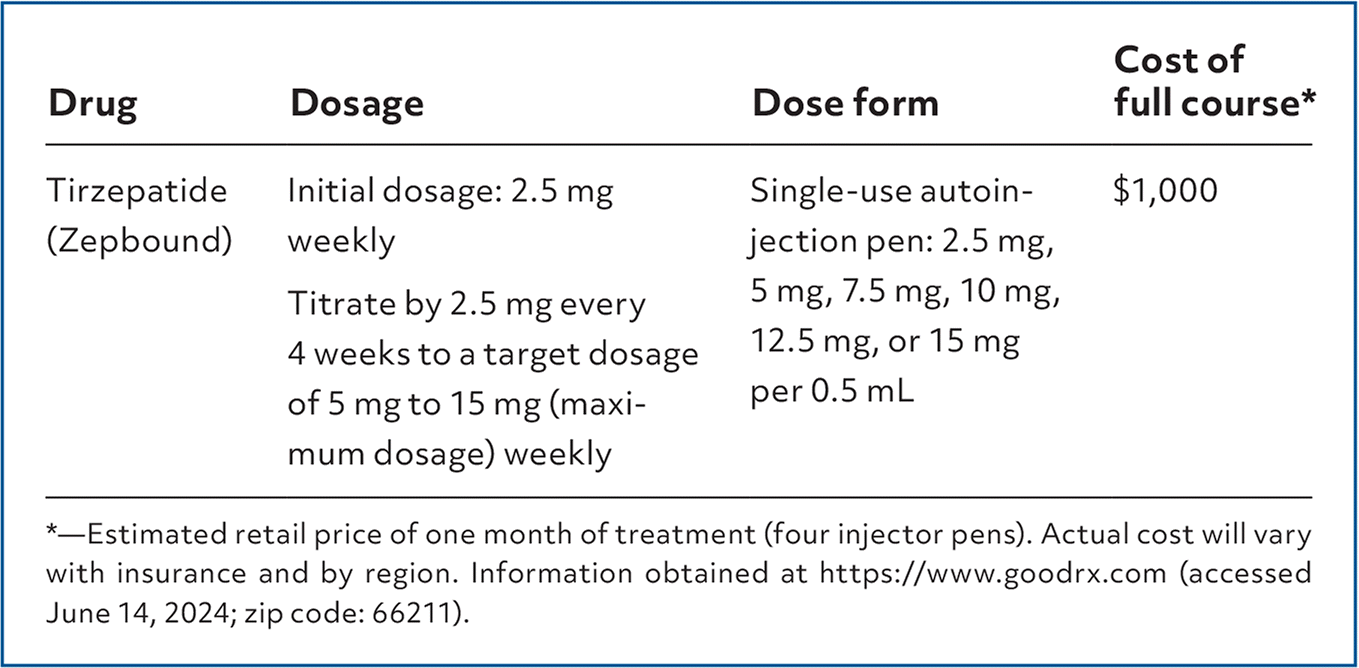
| Drug | Dosage | Dose form | Cost of full course* |
|---|---|---|---|
| Tirzepatide (Zepbound) | Initial dosage: 2.5 mg weekly Titrate by 2.5 mg every 4 weeks to a target dosage of 5 mg to 15 mg (maximum dosage) weekly | Single-use autoinjection pen: 2.5 mg, 5 mg, 7.5 mg, 10 mg, 12.5 mg, or 15 mg per 0.5 mL | $1,000 |
Subscribe
From $180- Immediate, unlimited access to all AFP content
- More than 125 CME credits/year
- AAFP app access
- Print delivery available
Issue Access
$59.95- Immediate, unlimited access to this issue's content
- CME credits
- AAFP app access
- Print delivery available
