
Am Fam Physician. 2024;110(3):313-314
Author disclosure: No relevant financial relationships.
Resmetirom (Rezdiffra), a thyroid hormone receptor-beta agonist, is conditionally approved for use in adults with noncirrhotic nonalcoholic steatohepatitis (NASH) with moderate to advanced fibrosis (stages F2 to F3) to reduce liver fat accumulation and to prevent progression of and reverse fibrosis when combined with diet and exercise.1 Resmetirom was approved using the U.S. Food and Drug Administration accelerated approval pathway on the basis of histologic data and may be granted final, traditional approval if the ongoing confirmatory trial verifies clinical outcome benefit.2
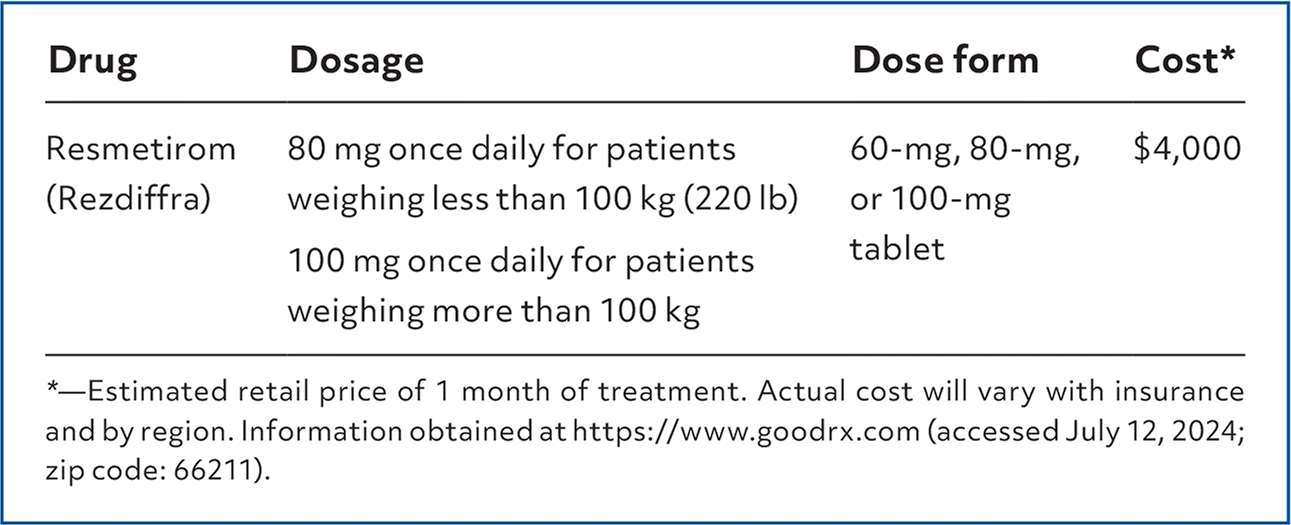
| Drug | Dosage | Dose form | Cost* |
|---|---|---|---|
| Resmetirom (Rezdiffra) | 80 mg once daily for patients weighing less than 100 kg (220 lb) 100 mg once daily for patients weighing more than 100 kg | 60-mg, 80-mg, or 100-mg tablet | $4,000 |
Subscribe
From $180- Immediate, unlimited access to all AFP content
- More than 125 CME credits/year
- AAFP app access
- Print delivery available
Issue Access
$59.95- Immediate, unlimited access to this issue's content
- CME credits
- AAFP app access
- Print delivery available
